November 25, 2020
November 25, 2020
On September 26, 2019, the European Medicines Agency (EMA) released an advice to Marketing Authorization Holders (MAH) of human medicines to review their drug products on possible presence of nitrosamines. At the same time FDA was investigating the presence of nitrosamines in certain drug products and as the consequence on September 1, 2020 the guideline for industry on control of nitrosamine impurities in human drugs was issued.
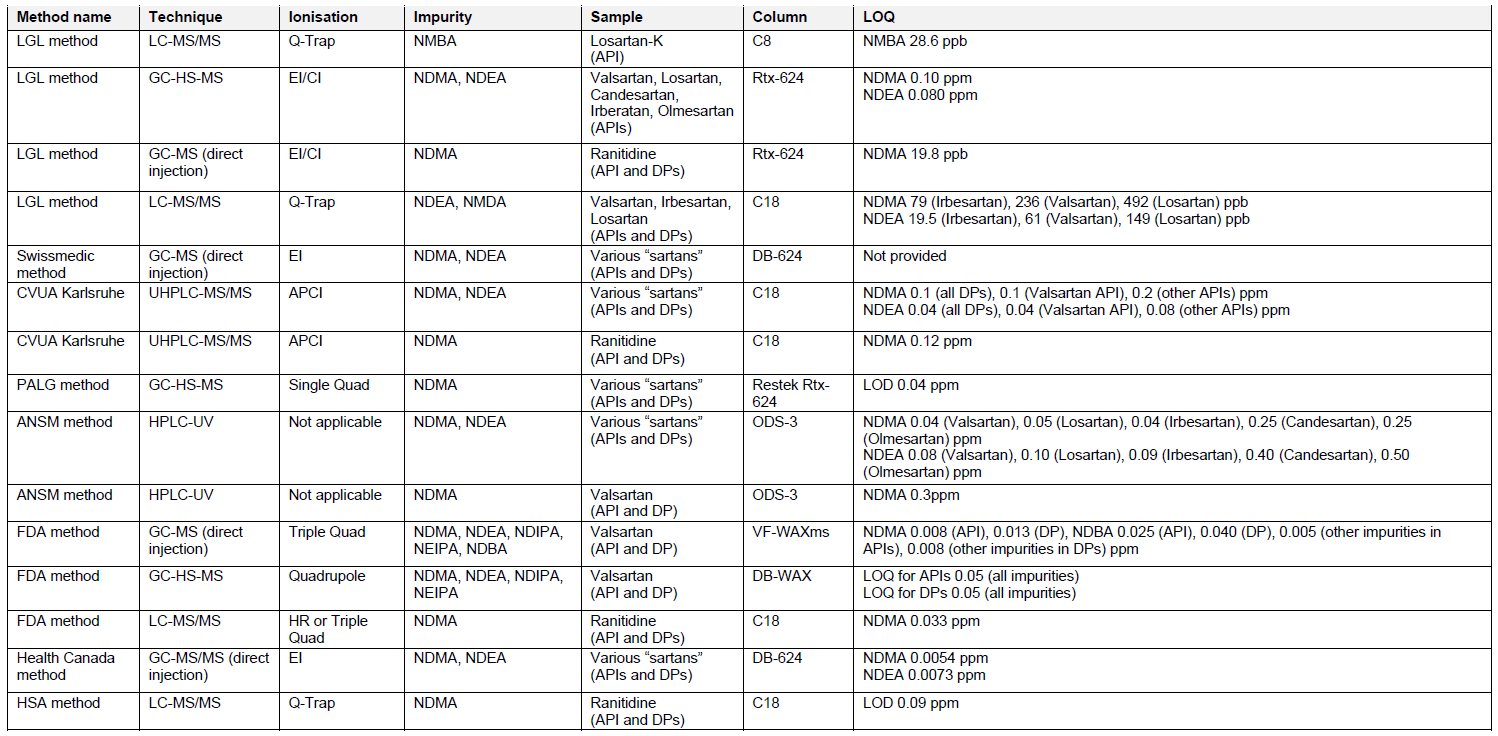
In 2018, a nitrosamine impurity was detected in a batch of valsartan, originating from a Chinese manufacturer. Valsartan is an active pharmaceutical ingredient (API) regulating blood pressure, which is formulated into a drug product for human use. The nitrosamine impurity found in valsartan was N-nitrosodimethylamine (NDMA). Nitrosamines were also detected in other angiotensin II receptor blockers, ranitidine, nizatidine and metformin, resulting in recalls of selected lots from the market and implementation of strict regulations to the manufacturing of these specific products.
Nitrosamines are not expected to form during the manufacture of the vast majority of medicines and they are commonly present in small amounts in drinking water and some food products. However, nitrosamines are potential carcinogenic substances. Long-term exposure above certain levels might increase the risk of cancer in humans. Therefore, marketing authorization holders should revise their medicinal products to identify and mitigate the risk of presence of nitrosamines. EMA currently requires the revision of all drug products containing chemically synthesized APIs and biological active substances. FDA expects the evaluation of chemicals and drug products at risk due to other factors listed in the respective guideline.
Currently recognized root causes of the presence of nitrosamine impurities in medicines are mostly associated with the manufacturing process in which nitrosamine precursors – nitrosable amines and nitrosating agents are present in the same or consecutive manufacturing steps. Nitrosamine impurities may be also introduced into the manufacturing process by contaminated, recycled, or recovered starting materials, reagents, solvents and catalysts. Potential carry-over, degradation profiles and use of certain packaging materials including printing inks ingredients associated risk should be evaluated as well.
All marketing authorization holders must review their human medicinal drug products on potential risk to containing nitrosamines impurities, following the applicable regional regulations. In EU the risk assessments will need to be completed by March 31, 2021 for chemical medicines and by July 1, 2021 for biological medicines. In US the risk assessments should be concluded by March 1, 2021.
Products identified of being at risk of containing nitrosamine impurities must be immediately tested using validated analytical test methods to quantify nitrosamine impurities, and authorities must be kept informed at all times. Adjustments in the manufacturing process to control levels of nitrosamine impurities and/or product specifications might be required. Within 3 years after the publication of the guidelines, the required changes to the drug product should be submitted, which is by September 1, 2023 for US and September 26, 2022 for EU with special extension for biologicals until July 1, 2023.
For more updated information and for assistance with next steps, please visit our page on the Control of Nitrosamine Impurities.
TAGS: Life Science Consulting
November 11, 2019
All Marketing Authorization Holders (MAH) of medicines for human use are facing what might feel for many, as a new requirement: review their drug products on the possible presence of nitrosamines....
May 4, 2020
EMA has extended the deadline to assess the risk of nitrosamine impurities to 31 March 2021. This decision was made based on the reports of the challenges encountered in meeting the original deadline...
March 22, 2021
Since September 26, 2019, all EU Marketing Authorization Holders (MAHs) of medicines for human use are facing what might be regarded as a new requirement: review their drug products on the possible...