January 28, 2015
January 28, 2015

Cleaning Validation and GMP reviews of those protocols are challenging. They become even more challenging at a Contract Manufacturing Organization (CMO) where compliance assessments to in-house Standard Operating Procedures (SOPs) as well as external regulatory guidelines is a requirement of in-house quality groups, clients, and of course regulators. Assessment of compliance to procedures can be revealing as well as educational. CMOs are required to develop and qualify analytical methodology for cleaning for the many active pharmaceutical ingredients (APIs) clients contract for manufacturing and validation services. One such challenging cleaning methodology component is swab recovery.
Attention is always immediately drawn to analytical methods with low recoveries. Recently, I reviewed a swab recovery method of an API with a particularly low recovery of under 50%. At first I thought this was a single outlying event perhaps due to the inherent properties of the API. However, I decided to look at other swab recoveries of similar APIs for comparison to confirm the outlier theory or to determine if there was an underlying trend. As there was an underlying trend, I resolved to identify various factors that may be contributing to the low swab recoveries.
Review of the post-cleaning swab methodologies revealed that a large percentage of APIs had swab recoveries well under the recommended acceptance value for various materials of construction (MOC). These particular APIs are compounds that can be generally categorized as manufactured biological protein analogues and therefore inherently soluble in water. After all, these compounds are intended as therapies for the human body. To contrast, these APIs are not small molecules which can be very insoluble in water and may require the use of harsh halogenated or petroleum based solvents for proper recovery.
Intuitively, these water-friendly proteins should be relatively easy to recover from various surfaces, usually in the range of 70-80% recovery. Based on the recovery data in Table 1 (below) this was not the case.
| Product Name | % Recovery | |||
|---|---|---|---|---|
| Stainless Steel | Glass | Silicone | EPDM | |
| API 1 | 80 | 77 | 56 | 86 |
| API 6 | 30 | 36 | 23 | 35 |
| API 8 | 32 | 31 | 25 | 29 |
| API 10 | 24 | 19 | 22 | 19 |
| API 12 | 14 | 13 | 16 | 23 |
| API 13 | 14 | 13 | 11 | 12 |
| API 14 | 79 | 76 | 87 | 90 |
| API 15 | 12 | 13 | 8 | 16 |
| API 16 | 33 | 30 | 21 | 24 |
| Cleaning Agent X | 49 | N/A* | 38 | N/A* |
N/A* - No data available for Glass and EPDM
Table 1: Swab recoveries for various surfaces
Swab recoveries less than 50% should be viewed as atypical, and require a different approach to determine what variables may be affecting swab recoveries. So what exactly are swab recoveries? Here are some terms to help clarify the issue:
With these terms in mind, what factors can contribute to low swab recoveries?
Let's consider:
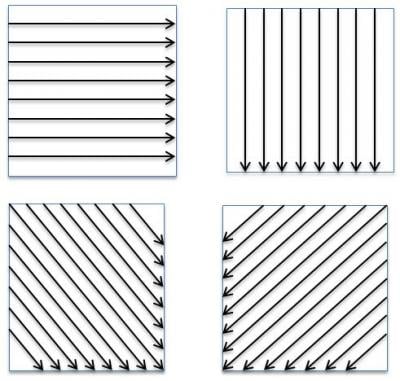
Figure 1: Swab direction
Swabbing has many different factors that can contribute to low recoveries. Taking a balanced methodical approach, which looks at data trends, swab selection, swab direction, extraction solvent, pH of extraction solvent and ionic characteristic can lead to higher swab recoveries and overall compliance. Strengthening areas where subjectivity may contribute to low swab recoveries is a proactive approach in eliminating variation and improving your swab recovery and compliance to your own procedures and policies.
Learn more about ProPharma's Cleaning Validation services.
Contact us to get in touch with one of our other subject matter experts for a customized presentation.
TAGS: Quality & Compliance Cleaning Validation Life Science Consulting
December 15, 2015
A common principle in Operational Qualification (OQ) studies is to challenge processes under worst case conditions. A "worst case" condition or set of conditions are generally those parameters...
February 4, 2013
While Standard Operating Procedures (SOP’s) are widely and rightly used to control processes, there is sometimes a tendency within organizations to go “reference crazy”. In a well-meaning attempt to...