March 1, 2022
March 1, 2022
In May 2022, the IVDD will be repealed by the European Committee, thereby ending the transition period. To ensure you're compliant with IVDR by that date, learn everything you need to know about the regulation including what it is and how to prepare for it.
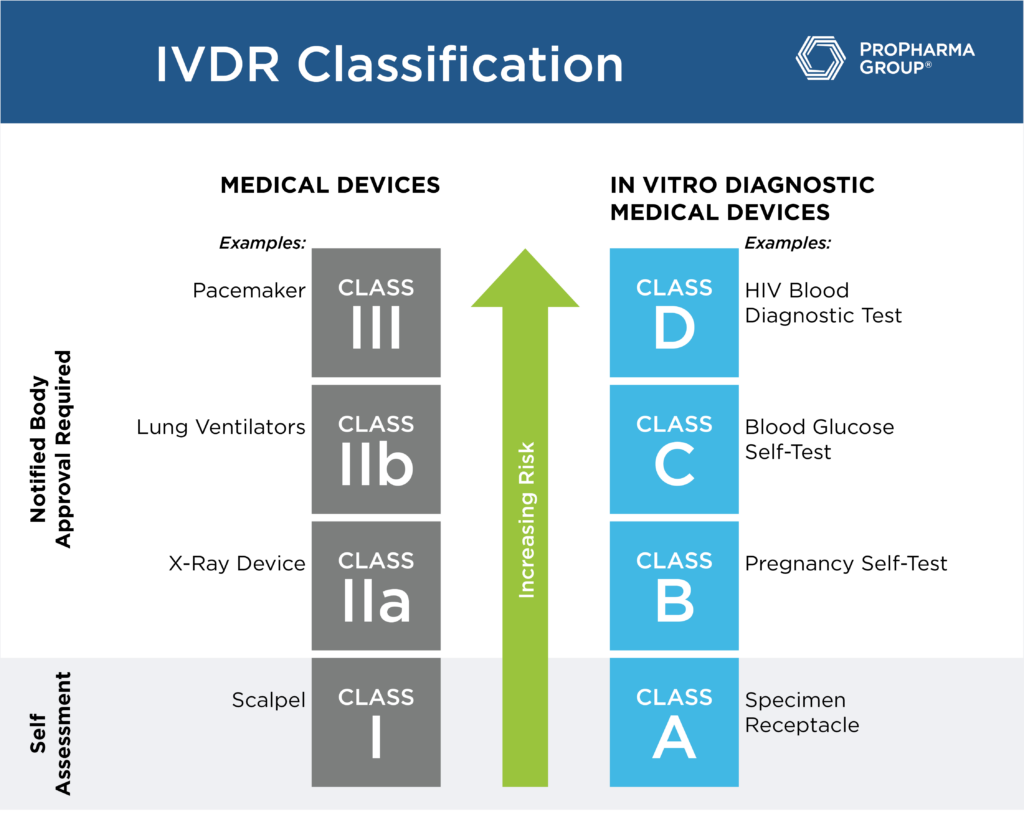
The In Vitro Diagnostic Regulation (IVDR) EU 2017/746 is the regulation for in vitro diagnostic medical devices that has been in the European market since 2017. The IVDR will create a "robust, transparent, and sustainable regulatory framework" that is recognized internationally while improving clinical safety and creating fair market access for manufacturers and healthcare professionals.
The IVDR replaces the EU Directive IVDD 98/79/EC. It is effective in all EU member states and EFTA states since 2017 without having to transfer the law into respective member states.
The IVDR initially came into effect on 25 May 2017, with a five-year transition period for manufacturers, which will end in May 2022. The transition period was intended to give manufacturers enough time to update their technical documentation and comply with the new IVDR regulation, which has more stringent requirements.
After 26 May 2022, new devices will have to comply with IVDR requirements to be placed on the European market. Under specific conditions including the fulfillment of prerequisite requirements of IVDR, products that already have IVDD certification by a Notified Body may be placed on the market until the end of their respective grace period has been reached, 25 May 2024.
How can you prepare in the final months before the IVDR date of application? How can you identify and plan for a transition from IVDD to IVDR and successfully manage the transition?
The deadline for EU IVDR is May 26, 2022. Use this readiness questionnaire to assess the current state of your progress.
IVDR raises the expectations for manufacturers and notified bodies alike through stricter requirements and more detailed regulations. Furthermore, it combines the state of the art of the directives and guidance documents into one aligned regulation.
While specific terms of IVDR are subject to change, some of the key changes for IVDR include:
IVDR presents new challenges for manufacturers of in vitro diagnostic medical devices. Meeting the regulation's new requirements will take considerable time and expertise. To prepare, manufacturers should:
The transition to the IVDR does not need to be overwhelming. Get expert guidance from the consultants at ProPharma Group. We help clients navigate the transition process to the IVDR efficiently and effectively, ensuring that you can maintain your current products in the marketplace and bring your new products to the market in the fastest possible manner. Contact our IVDR consultants today to learn more.
TAGS: IVDR Life Science Consulting
March 17, 2022
The compliance dates for the In Vitro Diagnostics Regulation (IVDR) are quickly approaching in May 2022. In this blog series, we discuss the final months before the IVDR date of application, how to...
February 2, 2022
Roadmap for Successful IVDR Transition: The compliance dates for the In Vitro Diagnostics Regulation (IVDR) will become effective on May 26, 2022. To help you with the IVDD to IVDR transition, we've...
February 17, 2022
The compliance dates for the In Vitro Diagnostics Regulation (IVDR) are quickly approaching (May 2022). In this blog series, we discuss the final months before the IVDR date of application along with...