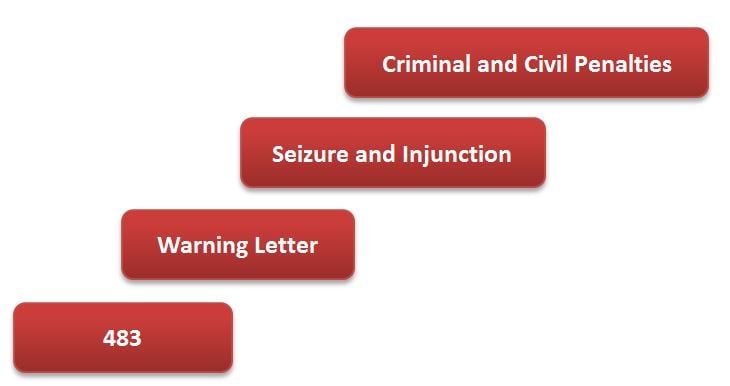
Starting with the issuance of a 483, the stepwise FDA enforcement process can be illustrated as follows:
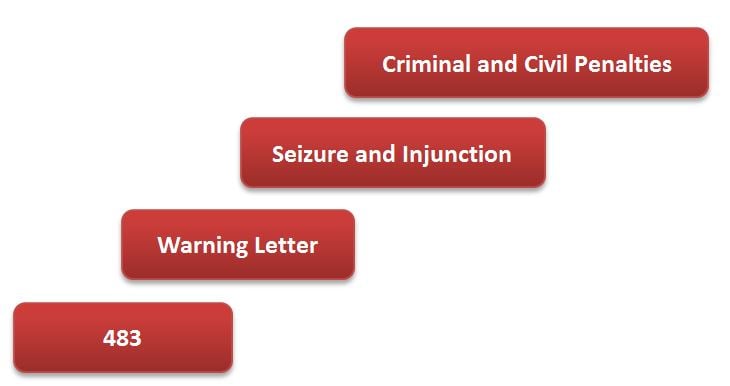
Given the seriousness of a Seizure and Injunction scenario, not to mention potential jail time if matters continue to go south after that, it is imperative that regulated companies never get beyond the Warning Letter phase.
Most QSR and cGMP Quality System professionals are aware that FDA posts warning letters on its website. Open to the public, the specific nonconformances and company responses related to an inspection’s findings and Form-483 issuance are displayed. This is certainly bad for business; reputations are sullied, shareholders are concerned and may consider litigation, supply chain partners are upset, audits become tenser, and so on.
However, despite the negative impact described, there is value in a periodic review of Warning Letters (note that one can actually subscribe and receive bi-monthly Warning Letter notifications from FDA). By studying these compliance gaps noted during an FDA inspection, one can address ways to avoid similar non-compliant practices in one’s company.
Here is an example:
Violation: failure to have sufficient personnel with the necessary education, background, training, and experience to assure that all activities required by 21 CFR 820 are correctly performed, as required by 21 CFR 820.25(a). For example:
a. The job description for the Director of Quality Systems requires that the person have a Bachelor of Science/Technical/or Engineering discipline. The person holding the position does not have this type of degree, but rather a Business Administration degree.
b. The person holding the Regulatory Affairs Manager position lacks the minimum of 5 years of regulatory experience required in the job description.
c. The person holding the Quality Control Supervisor position lacks the required Bachelor degree in science or the alternative five to eight years’ experience in Quality Control.
d. The person holding the Calibration Coordinator position lacks the required Bachelor degree and the four years of relevant experience.
Obviously this is an example of a company not following their own SOPs; however, if you are an outsider looking in, you may want to verify that your personnel’s qualifications are aligned with their job descriptions to avoid a similar fate.
Learn more about ProPharma Group's Compliance services.
Contact us to get in touch with our subject matter experts for a customized Compliance presentation.