March 12, 2013

March 12, 2013

Unexpected events often happen in the everyday world. However, in the regulated world great efforts are made to minimize the severity and frequency of unexpected events. Yet, despite best efforts, these events still occur. Therefore, a strong investigation system is needed to find the root cause of the event and identify corrective and preventive actions (CAPA). Implementation of effective CAPAs and strong effectiveness checks completes the CAPA system and bring the unexpected event to a close. But what are the building blocks for a strong investigation? There are three key steps needed to conduct a strong investigation.
When first initiating an investigation, ensure the entire scope of the event is grasped. Understand what happened, where it happened, when it happened, who was involved, the part and lot numbers of materials involved, what steps of the process were being performed at the time, what SOPs govern the process, what corrections were completed immediately, including containment of any potentially adulterated product, any testing that was performed, including results, and the initial impact assessment of the event which should detail product impact. Each of these substantiated findings will provide the foundation necessary to begin the investigation. A good tool to use is to develop a checklist of all necessary initial information and go through that list at the start of each investigation. An example list is shown below.
This step is important because not only will it provide valuable information about the event that may not be in the initial event summary, but will also allow an opportunity to build a relationship with personnel that can benefit future investigations. Discussions with line operators, supervisors, and management will all bring different pieces of information to the table. These discussions will bring more detail to the event as well as provide the groundwork for potential CAPA. With each interaction, ask the employee how the system could be improved in their opinion. While these suggestions might not be suitable for potential CAPA, they will still provide valuable information regarding the employee's view of manufacturing and quality system effectiveness. This step is sometimes difficult due to gowning requirements or timing of shifts, but can be one of the most valuable steps in the investigation.
This step is especially important when working on sites that are under consent decree or that have a less than robust quality system. Understanding how a process should run, from the company's perspective, will provide insight that can prove valuable, not only for identifying the root cause, but also for recommending effective CAPAs. It is not an uncommon practice, especially for companies with deficient quality systems, to blame employees for unexpected events. This allows them to identify an "easy" root cause and close out investigations with simple CAPAs such as counseling or training. Process improvement experts rarely blame people (because people generally try to do the right thing) and instead, focus on the process. In other words, the process allowed the person to fail. The key to a strong investigation is understanding what should have happened as well as what actually happened. Analyzing the procedures can often identify gaps in a process that management or line personnel may take for granted.
There are many tools and techniques for conducting investigations, but having a strong foundation for the investigation is the most important component. Establishing a strong foundation for the investigation will lead to a solid, defendable, and justifiable remediation effort.
TAGS: Compliance Quality & Compliance Life Science Consulting
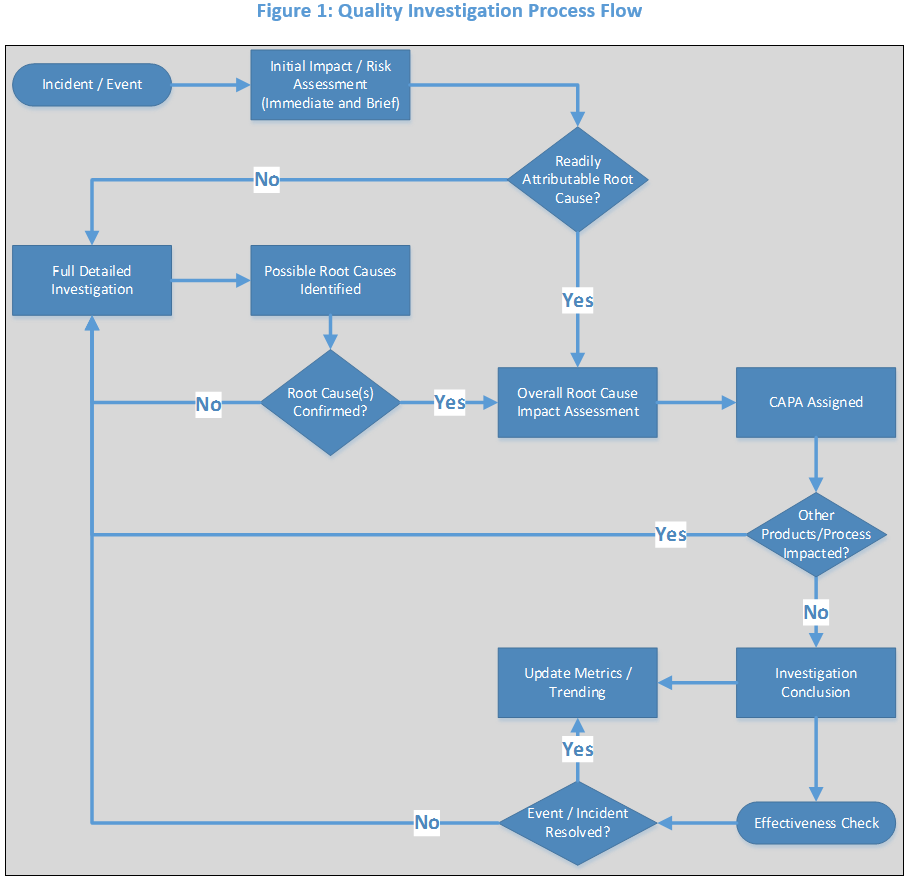
July 6, 2017
In 2016, the FDA issued hundreds of 483 observations across the Drug and Device industries for failing to thoroughly review or investigate issues. This topic consistently hits the top five most...
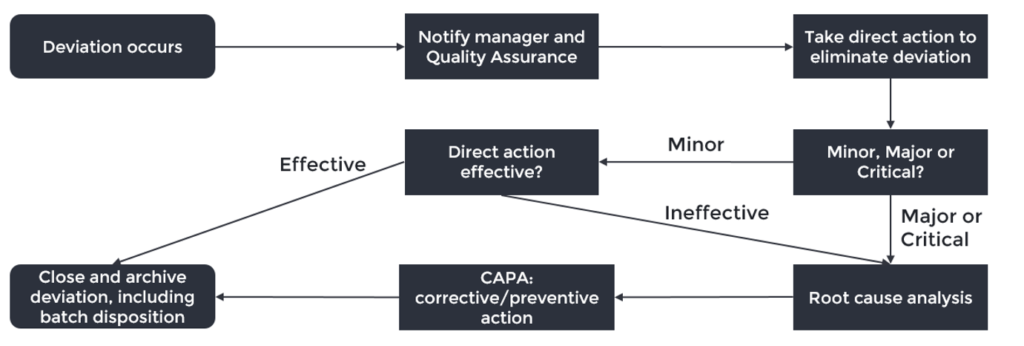
June 25, 2019
There are plenty of guidelines and instructions on implementing a deviation system in a pharmaceutical/medical device company. However, there is a big difference between theory and practice when it...
May 20, 2021
In early 2020, while COVID-19 was wreaking havoc on public health and safety, the FDA took the unprecedented step of postponing domestic and foreign inspections. The FDA’s risk-based inspection...