
Regulatory Sciences
FDA Releases Draft Guidance for Industry on Clinical Pharmacology Considerations for Human Radiolabeled Mass Balance Studies
The FDA’s Office of Clinical Pharmacology within the Office of Translational Sciences released a new draft guidance document that, for the first time, clearly addresses the FDA’s recommendations and...

Investigating Out-of-Specification (OOS) Test Results for Pharmaceutical Production - Level 2 revision
Guidance for Industry May 2022 Today, FDA is announcing revisions to the 2006 guidance “Investigating Out-of-Specification (OOS) Test Results for Pharmaceutical Production.” Specifically, this...

Meet the Expert: Steven Silverman
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...

Regulatory Sciences
FDA's Expedited Programs Explained
In order to incentivize the development of therapies (drugs biologics) to fill unmet medical needs for treatment of serious conditions, the FDA has developed various programs to expedite drug...
Battle of the Regulators: Comparing FDA’s Accelerated Approval and EMA’s Conditional Marketing Authorisation
The Food and Drug Administration (FDA) and the European Medicines Agency (EMA) offer various pathways that can expedite the development and review of new therapies to treat serious or...
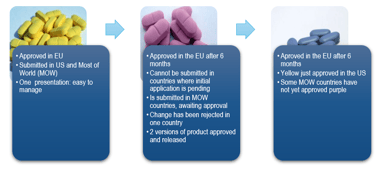
CMC Regulatory Dossier Compliance: A GMP Requirement
Maintaining compliance in the dynamic regulatory Chemistry, Manufacturing and Controls (CMC field can be quite a challenge. A CMC regulatory dossier compliance assessment is a critical component and...
Meet the Expert: Marshall Scicchitano
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...
Regulatory Sciences
FDA’s Breakthrough Therapy Designation vs PRIority MEdicines (PRIME) Application in Europe
What are Breakthrough Therapy Designation and PRIority MEdicines (PRIME) Applications? The advancement of modern medicine, and the accessibility of researched and regulated medication, has greatly...
Regulatory Strategy for Clinical Trials in the European Union
Setting up a clinical trial in the European Union (EU) has historically been an expensive and time-consuming business. With 27 individual member states each requiring its own review and approval, it...
Is it Time to Sharpen Your Target Product Profile (TPP)?
Is it Time to Sharpen Your Target Product Profile (TPP)? We’ve heard it countless times: "Fail early and live to fight another day." "Preserve capital, human resources, and energy for a project with...
Regulatory Sciences
Understanding Bioequivalence and Product-Specific Guidances
The FDA regularly issues new and revised product-specific guidances to facilitate the availability of generic drugs and assist the generic pharmaceutical industry with identifying the most...

Meet the Expert: Daniel Solorio
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...

European Expedited Regulatory Programs Explained
Do you really know how to accelerate the approval of your innovative product in Europe? The FDA's incentives for promising new medicines are widely known. Accelerated approval, priority review, fast...
Regulatory Sciences
Four Benefits of Requesting an FDA Pre-IND Meeting
Although not required, a Pre-IND Meeting is a critical milestone, one that is highly recommended by the FDA. The goal of the meeting is to receive general agreement from FDA that your drug...
Regulatory Sciences
Critical Issues in Drug Development: Don't Get Derailed in Developmental Efforts
Pharmaceutical companies spend massive amounts of money on drug development. Regardless of a firm’s size, the investment is substantial; the smallest error can have catastrophic consequences,...
Regulatory Sciences
How to Present Scientific Data That Generates Regulatory Approvals
Today’s patients benefit from having access to the safest, most advanced pharmaceutical systems around the world. This is because of the work done by the various regulatory bodies who govern the...