
Regulatory Sciences
UK Paediatric Investigational Plans – what do you need to know?? …and how is it all working in practice??
If a marketing authorisation is planned to be submitted in England, Scotland, and Wales (GB), an MHRA-approved paediatric investigational plan (PIP) is required. Up until January 1, 2021, PIPs were...

EMA: Big data use for public health: publication of Big Data Steering Group workplan 2022-25
News July 28, 2022 The Big Data Steering Group set up by EMA and the Heads of Medicines Agencies (HMA) has published its third workplan that sets key actions to be delivered between 2022–25. The new...
How to Fast-Track medicine approval in the UK with the MHRA’s Innovative Licensing and Access Pathway (ILAP)
What is ILAP? What benefits does ILAP provide? How do you access it? With the dust of Brexit settling, the question on most people’s lips (well, those of us in the healthcare sector anyway!) was:...

Regulatory Sciences
5 Benefits of Receiving EU PRIME Designation for Medicine Developers
What it is, why it matters, how you can apply and how we can help. What is the PRIME Scheme? You might be forgiven if you don’t know what the PRIority MEdicines (PRIME) scheme is; but if you are in...
%20inspection%20procedures.jpeg?width=384&height=256&name=EMA%20Good%20clinical%20practice%20(GCP)%20inspection%20procedures.jpeg)
EMA Good clinical practice (GCP) inspection procedures
The Good Clinical Practice (GCP) Inspectors Working Group has developed procedures for the coordination, preparation, conduct and reporting of GCP inspections requested by the European Medicines...
Orphan Drug Designations in the US and EU
What is an Orphan Drug Designation? The Orphan Drug Designation (ODD) program in both the United States (U.S.) and European Union (E.U.) qualifies sponsors to receive potential incentives to develop...
Regulatory Sciences
Global regulators agree on key principles on adapting vaccines to tackle virus variants
On 30 June, regulators from around the world discussed emerging evidence to support adaptation of COVID-19 vaccines as the SARS-COV-2 virus continues to evolve during a workshop co-chaired by the...

Regulatory Sciences
Clinical Pharmacology Considerations for Oligonucleotides
For the first time, the FDA has issued a draft guidance for industry on “Clinical Pharmacology Considerations for the Development of Oligonucleotide Therapeutics”. Oligonucleotides are short single...
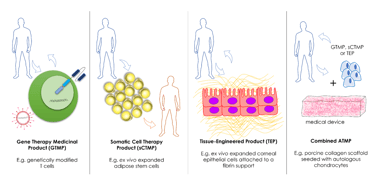
How to Leverage EMA’s ATMP Classification
Advanced therapy medicinal products (ATMPs) have emerged as ground-breaking therapies for rare diseases and other conditions with unmet clinical needs. As of 2022, sixteen ATMPs have been approved by...
Maximising on Scientific Advice Procedures in Europe
A unique opportunity to interact with medicine regulators in Europe Are you considering requesting scientific advice in Europe? We can help you navigate the various procedures within the European...
EMA adopts first list of critical medicines for COVID-19
News On 7 June 2022, EMA's Medicines Shortages Steering Group (MSSG) adopted the list of critical medicines for the COVID-19 public health emergency. The medicines included in the list are authorised...

Understanding ICH M10 “Bioanalytical Method Validation and Study Sample Analysis"
The final version of ICH M10 “Bioanalytical Method Validation and Study Sample Analysis” was adopted on May 24, 2022. This is the harmonized guideline which has been ratified by participating...

FDA publishes MAPP 5223.6, Assessment of the User Interface of a Drug-Device Combination Product Submitted in a Pre-ANDA Communication or an ANDA
Today, FDA published a new Manual of Policies and Procedures (MAPP), “Assessment of the User Interface of a Drug-Device Combination Product Submitted in a Pre-ANDA Communication or an ANDA (5223.6).”...

Regulatory Sciences
Compounded drug search added to FDA’s NDC directory webpage
FDA recently added a search function to the National Drug Code (NDC) Directory webpage for human drugs compounded by outsourcing facilities that assign NDC numbers to their products. This update was...

EMA appoints Chief Medical Officer
June 1, 2022 Steffen Thirstrup has been appointed as Chief Medical Officer of EMA. In this role, he will provide scientific leadership across EMA and its scientific committees to reinforce the...
Regulatory Sciences
How to Avoid Common Pitfalls in the Development of Biosimilars
As many blockbuster biologicals face the expiration of their patents, so-called "patent cliff", many biotech businesses direct their attention to the field of biosimilars. The development of...