Clinical Research Solutions
Ban on Titanium Dioxide (E171) on the EU Food Market: What Are the Consequences for Medicines?
Use of Titanium Dioxide in the EU Food Market In 2021, the European Food Safety Authority (EFSA) investigated the safety of the white coloring agent titanium dioxide (TiO2) and concluded that the...
Regulatory Sciences
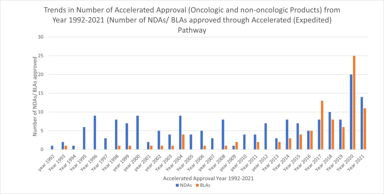
FDA Accelerated Approval Pathway: A Potential Missed Opportunity for Sponsors
The accelerated approval provisions of FDASIA in section 506(c) of the FD&C Act provide that FDA may grant accelerated approval to: . . . a product for a serious or life-threatening disease or...
Clinical Research Solutions
What You Need to Know About Developing Vaccines
An unlikely beacon of hope from the otherwise disastrous Covid pandemic, may come in the form of renewed attention towards approaches to vaccine development. The Importance of Vaccines The...

Clinical Research Solutions
Prescription Drug User Fee Act (PDUFA) VII and Type D Meetings: A New Mechanism for Interacting with FDA
For those who have been awaiting Congressional reauthorization of PDUFA, the wait is over. On September 30, 2022, the President signed into law the FDA User Fee Reauthorization Act of 2022. We...

Clinical Research Solutions
What You Need to Know About CBER Pre-IND Meetings
The FDA provides several opportunities to hold meetings with Sponsors to gain clarification and agreement on the development of medicinal products. At the preliminary stages of development, one such...
Clinical Research Solutions
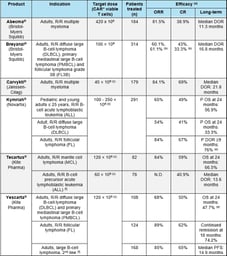
CAR-T Cells: Challenges, Lessons Learned, and Guidance for the Clinical Development
It comes as no surprise to any pharmaceutical or biotech company that planning the clinical development of CAR-T cells is an extremely challenging endeavor: high efficacy is expected in each targeted...

Clinical Research Solutions
How to Comply with the Nitrosamine Regulations for Your New Drug Product Marketing Applications
Introduction: Are you in the development phase for your medicinal product? Have you assessed your manufacturing processes with respect to the requirements for investigating the potential presence of...
Clinical Research Solutions
FDA Designates Empirically Based Bayesian Emax Models for Dose Finding as ‘Fit-For-Purpose’
FDA Designates Empirically Based Bayesian Emax Models for Dose Finding as ‘Fit-For-Purpose’: On August 5, 2022, the U.S. Food and Drug Administration (FDA) designated ‘Empirically Based Bayesian Emax...

Clinical Research Solutions
FDA Issues FY2021 Report on the State of Pharmaceutical Quality
FDA Issues FY2021 Report on the State of Pharmaceutical Quality: The Office of Pharmaceutical Quality (OPQ) within FDA’s Center for Drug Evaluation and Research has published the fiscal year 2021...
Clinical Research Solutions
FDA Publishes Responses to Good Clinical Practice Inquiries
FDA Publishes Responses to Good Clinical Practice Inquiries: FDA oversees clinical trials to ensure they are designed, conducted, analyzed and reported according to federal law and FDA’s regulations....