Quality & Compliance
What is Software Quality Assurance (SQA) and Do You Need it For Your Business?
If your only method of quality assurance is software testing, you're wasting valuable time and resources. Software Quality Assurance (SQA) is integrated into your software development life cycle to...
Quality & Compliance
Meet the Expert: Gary Hyde
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...

Quality & Compliance
Project Management and a Successful Reduction in Investigations Backlog: The Beauty and the Beast
Many of us have been faced with this beast that needs taming: a regulatory agency has conducted an inspection of your facility. Their observation is that the backlog of investigations at your site is...
Quality & Compliance
5 Steps to CMO Identification and Selection for Cell and Gene Therapies
After assuring clinical validity, finding and managing the right contract manufacturing organizations (CMOs)/contract development manufacturing organizations (CDMOs) is a Sponsor's major concern when...
Quality & Compliance
Why is Process Optimization so Important in Cell and Gene Therapy Product Development?
A recent survey of experts from 145 cell and gene therapy (CAGT) companies revealed the ability to appropriately optimize the manufacturing process as their top concern. Red flags have been raised...

Quality & Compliance
12 Critical Questions and Answers for a Successful Tech Transfer
12 Critical Questions and Answers for a Successful Tech Transfer Now, more than ever, companies are transferring products and processes from one site to another, often facing pressures on time,...
Quality & Compliance
Is Your Technical Transfer Process Aligned with Process Validation Requirements?
There has been a lot of discussion recently concerning process validation and technology transfer, including utilizing virtual technology transfers to quickly move products through the development...
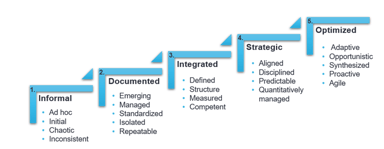
Quality & Compliance
Improve Quality Using an Organizational Maturity Model
If this is your first introduction to an Organizational Maturity Model (OMM), you may have a few questions. What is an OMM? What are some common obstacles I might face when implementing an OMM? How...
Regulatory Sciences
Understanding EMA and FDA Regulations on Nitrosamine Control
On September 26, 2019, the European Medicines Agency (EMA) released an advice to Marketing Authorization Holders (MAH) of human medicines to review their drug products on possible presence of...
Quality & Compliance
Are Your Compliance Obligations Being Properly Upheld? Avoid This Common Outsourcing Mistake!
Over the past several decades, the traditional approach to drug development and manufacturing has expanded to include the outsourcing of a range of functions from product development and testing, to...
Unlocking Success with Clinical Trial Safety Monitoring During a Pandemic
Earlier this year I wrote to you about US FDA March 2020 issuance of a new guidance for industry, Investigators, and Institutional Review Boards regarding the conduct of clinical trials during the...

Quality & Compliance
Uncover Opportunities for Improvement with an Annual Product Review
The Annual Product Review (APR), also known as the Annual Product Quality Review (APQR), is required for marketed products in an FDA-regulated environment. You may ask, "Why would I want to perform...
Quality & Compliance
EudraLex Volume 4, Annex 1 Update: What You Need to Know
EudraLex Volume 4, Annex 1 provides guidance for the manufacturing of sterile medicinal products that are intended for the European market. It has been updated several times, with the latest revision...
6 Tips to Prepare Your Medical Cannabis Facility for Inspection
You may be considering building a new facility for growing, harvesting, and processing medical cannabis, or perhaps you have an existing facility and want to export to the European Union. What should...
Quality & Compliance
Virtual GCP Auditing: Your Questions Answered!
How quickly the auditing landscape has changed! Less than one year ago if ProPharma were asked to perform a clinical audit on your firm’s behalf, we would reply with "when, what, and where?" Today...
Quality & Compliance
Key Takeaways: New Draft Guidance on Cannabis and Clinical Research
On July 21st, 2020, the FDA released a draft guidance document for developers of cannabis and cannabis derived compounds, aptly titled “Cannabis and Cannabis Derived Compounds: Quality Considerations...