Clinical Research Solutions
Is Your Pharmacovigilance Team Ready for Brexit?
As we approach the final months of 2020, the pharmaceutical world begins, once again, to focus its thoughts on the impact of Brexit, not least in the world of pharmacovigilance. Of course, the UK has...
Clinical Research Solutions
EMA Vaccine Applications for COVID-19 Explained
Preventive vaccines are a special kind of medicinal product, as they are administered before a disease instead of during. You are probably familiar with preventive vaccines from your own or your...
Regulatory Sciences
A Guide to Understanding Long Term Follow-Up for Gene Therapy Clinical Trials
When conducting a clinical trial, there are many aspects sponsors need to be aware of with regards to clinical safety, product efficacy, and the ability to bring treatments through the multiple...
Clinical Research Solutions
FDA Guidance for Non-COVID-19 Related Clinical Trials During the Pandemic
In March 2020, the FDA published a guidance entitled “Conduct of Clinical Trials of Medical Products during COVID-19 Public Health Emergency - Guidance for Industry, Investigators, and Institutional...
Clinical Research Solutions
Clinical Trials in the Midst of COVID-19, Part One: European Medicines Agency (EMA)
Disclaimer As of April 23, 2020 Each health authority within the EU has information regarding COVID-19 (coronavirus) on its website. In this blog we will try to guide you through some of these...
Clinical Research Solutions
Rapid and Responsive Implementation the Right Way
During the COVID-19 global pandemic, Medical Information (MI) and Pharmacovigilance (PV) teams are providing an extremely valuable service to deliver current and accurate product information to...
Clinical Research Solutions
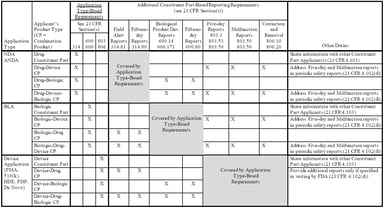
Understanding the New Combination Product PMSR Guidance Documents and Impact on Industry
On March 20, 2018, the US Food and Drug Administration (FDA) released two new guidance documents to help companies comply with the December 20, 2016 final rule establishing postmarketing safety...