
Clinical Research Solutions
USP and FDA Propose Updates to Good Storage and Distribution Practices
Updates have been announced by FDA and for USP <1079>. In this blog we cover these changes. USP USP <1079> has a series of chapters on Good Storage and Distribution Practices. Chapter <1079> applies...
Clinical Research Solutions
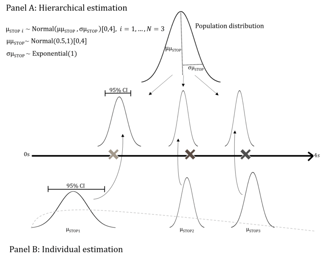
FDA Designates Empirically Based Bayesian Emax Models for Dose Finding as ‘Fit-For-Purpose’
FDA Designates Empirically Based Bayesian Emax Models for Dose Finding as ‘Fit-For-Purpose’: On August 5, 2022, the U.S. Food and Drug Administration (FDA) designated ‘Empirically Based Bayesian Emax...

Clinical Research Solutions
FDA Issues FY2021 Report on the State of Pharmaceutical Quality
FDA Issues FY2021 Report on the State of Pharmaceutical Quality: The Office of Pharmaceutical Quality (OPQ) within FDA’s Center for Drug Evaluation and Research has published the fiscal year 2021...
Clinical Research Solutions
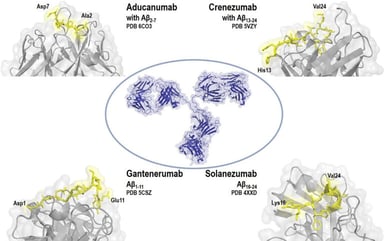
FDA Publishes Responses to Good Clinical Practice Inquiries
FDA Publishes Responses to Good Clinical Practice Inquiries: FDA oversees clinical trials to ensure they are designed, conducted, analyzed and reported according to federal law and FDA’s regulations....
Clinical Research Solutions
Orphan Designation of ATMPs for Rare Diseases: MPS II Case Study
Orphan Designation of ATMPs for Rare Diseases: MPS II Case Study Many advanced therapy medicinal products (ATMPs) in development in the EU are for rare diseases and conditions. Since the...

Clinical Research Solutions
FDA Pathways to Medical Device Approval
Commercializing your medical device in the US market often requires submitting a marketing application to the FDA to become an FDA Approved or Cleared Medical Device. The content of your FDA...

Clinical Research Solutions
FDA Solicits Feedback on ANDA Submissions – Amendments to ANDAs Under GDUFA Guidance, Appendix A
Ahead of this year’s reauthorization of the Generic Drug User Fee Amendments (GDUFA) , FDA has established a docket to solicit comments on the content of Appendix A in the July 2018 guidance for...
Clinical Research Solutions
FDA Publishes Complex Generics News Resource
Today the FDA is publishing a new web page to share the most recent FDA actions and activities related to complex generics. This new resource is part of FDA’s continued commitment to ensuring...

Clinical Research Solutions
FDA Recognizes August as National Immunization Awareness Month
National Immunization Awareness Month provides us an opportunity to think about how far the development and advancement of immunization science has come, and its impact on public health. The U.S....

Clinical Research Solutions
FDA publishes product-specific guidances to facilitate generic drug development
Today, FDA published a new batch of product-specific guidances (PSGs). PSGs provide recommendations for developing generic drugs and generating the evidence needed to support abbreviated new drug...