
Quality & Compliance
USP and FDA Propose Updates to Good Storage and Distribution Practices
Updates have been announced by FDA and for USP <1079>. In this blog we cover these changes. USP USP <1079> has a series of chapters on Good Storage and Distribution Practices. Chapter <1079> applies...
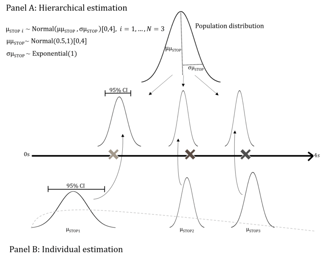
FDA Designates Empirically Based Bayesian Emax Models for Dose Finding as ‘Fit-For-Purpose’
FDA Designates Empirically Based Bayesian Emax Models for Dose Finding as ‘Fit-For-Purpose’: On August 5, 2022, the U.S. Food and Drug Administration (FDA) designated ‘Empirically Based Bayesian Emax...

FDA Issues FY2021 Report on the State of Pharmaceutical Quality
FDA Issues FY2021 Report on the State of Pharmaceutical Quality: The Office of Pharmaceutical Quality (OPQ) within FDA’s Center for Drug Evaluation and Research has published the fiscal year 2021...
FDA Publishes Responses to Good Clinical Practice Inquiries
FDA Publishes Responses to Good Clinical Practice Inquiries: FDA oversees clinical trials to ensure they are designed, conducted, analyzed and reported according to federal law and FDA’s regulations....
Regulatory Sciences
Orphan Designation of ATMPs for Rare Diseases: MPS II Case Study
Many advanced therapy medicinal products (ATMPs) in development in the EU are for rare diseases and conditions. Since the establishment of the Advanced Therapies Regulation in 2008 in the European...
Regulatory Sciences
FDA Pathways to Medical Device Approval
Commercializing your medical device in the US market often requires submitting a marketing application to the FDA to become an FDA Approved or Cleared Medical Device. The content of your FDA...

Regulatory Sciences
FDA Solicits Feedback on ANDA Submissions – Amendments to ANDAs Under GDUFA Guidance, Appendix A
Ahead of this year’s reauthorization of the Generic Drug User Fee Amendments (GDUFA), FDA has established a docket to solicit comments on the content of Appendix A in the July 2018 guidance for...
Regulatory Sciences
FDA Publishes Complex Generics News Resource
Today the FDA is publishing a new web page to share the most recent FDA actions and activities related to complex generics. This new resource is part of FDA’s continued commitment to ensuring...

Regulatory Sciences
FDA Recognizes August as National Immunization Awareness Month
National Immunization Awareness Month provides us an opportunity to think about how far the development and advancement of immunization science has come, and its impact on public health. The U.S....

Regulatory Sciences
FDA publishes product-specific guidances to facilitate generic drug development
Today, FDA published a new batch of product-specific guidances (PSGs). PSGs provide recommendations for developing generic drugs and generating the evidence needed to support abbreviated new drug...

Regulatory Sciences
UK Paediatric Investigational Plans – what do you need to know?? …and how is it all working in practice??
If a marketing authorisation is planned to be submitted in England, Scotland, and Wales (GB), an MHRA-approved paediatric investigational plan (PIP) is required. Up until January 1, 2021, PIPs were...

Regulatory Sciences
Clinical Pharmacology Considerations for Oligonucleotides
For the first time, the FDA has issued a draft guidance for industry on “Clinical Pharmacology Considerations for the Development of Oligonucleotide Therapeutics”. Oligonucleotides are short single...

Understanding ICH M10 “Bioanalytical Method Validation and Study Sample Analysis"
The final version of ICH M10 “Bioanalytical Method Validation and Study Sample Analysis” was adopted on May 24, 2022. This is the harmonized guideline which has been ratified by participating...

FDA publishes MAPP 5223.6, Assessment of the User Interface of a Drug-Device Combination Product Submitted in a Pre-ANDA Communication or an ANDA
Today, FDA published a new Manual of Policies and Procedures (MAPP), “Assessment of the User Interface of a Drug-Device Combination Product Submitted in a Pre-ANDA Communication or an ANDA (5223.6).”...

Regulatory Sciences
Compounded drug search added to FDA’s NDC directory webpage
FDA recently added a search function to the National Drug Code (NDC) Directory webpage for human drugs compounded by outsourcing facilities that assign NDC numbers to their products. This update was...
FDA Issues Final Guidance on Safety Considerations for Container Labels and Carton Labeling Design to Minimize Medication Errors
FDA has issued the final guidance titled “Safety Considerations for Container Labels and Carton Labeling Design to Minimize Medication Errors.” The recommendations for prescription drug and...