
Clinical Research Solutions
Enhancing CRO Capabilities with Independent Physician Services
The involvement of Medical Monitoring (MM) and Data Safety Monitoring Boards (DSMB) in trials has been somewhat unknown to individuals outside of clinical circles. That is, until media attention...
Clinical Research Solutions
Post-COVID Challenges with Investigation Backlog: Are You Ready for the Inevitable?
In early 2020, while COVID-19 was wreaking havoc on public health and safety, the FDA took the unprecedented step of postponing domestic and foreign inspections. The FDA’s risk-based inspection...
Clinical Research Solutions
FDA Guidance on Gene Therapy Products: RCR Testing and Monitoring
Gene therapy holds the promise of curing severe genetic diseases at the genetic level rather than merely treating the symptoms as is accomplished using conventional small-molecule drug therapies. In...
Quality & Compliance
Understanding Responsible Person and Wholesaler Dealers Authorization
Wholesaling is a key factor for drug manufactures and Marketing Authorization Holders (MAH) to ensure supply of pharmaceutical products to their customers at pharmacies and hospitals. Wholesaling of...

Clinical Research Solutions
Meet the Expert: Gary Hyde
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...

Clinical Research Solutions
Project Management and a Successful Reduction in Investigations Backlog: The Beauty and the Beast
Many of us have been faced with this beast that needs taming: a regulatory agency has conducted an inspection of your facility. Their observation is that the backlog of investigations at your site is...
Clinical Research Solutions
5 Steps to CMO Identification and Selection for Cell and Gene Therapies
After assuring clinical validity, finding and managing the right contract manufacturing organizations (CMOs)/contract development manufacturing organizations (CDMOs) is a Sponsor's major concern when...
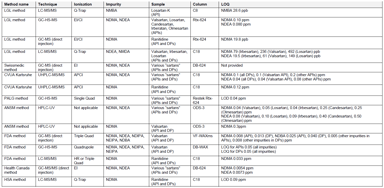
Clinical Research Solutions
Analytical Methods for the Investigation of Carcinogenic Nitrosamines in APIs and Drug Products
Since September 26, 2019, all EU Marketing Authorization Holders (MAHs) of medicines for human use are facing what might be regarded as a new requirement: review their drug products on the possible...
Clinical Research Solutions
Why is Process Optimization so Important in Cell and Gene Therapy Product Development?
A recent survey of experts from 145 cell and gene therapy (CAGT) companies revealed the ability to appropriately optimize the manufacturing process as their top concern. Red flags have been raised...
