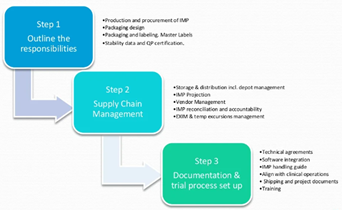
Quality & Compliance
15 Days of Panic: You Received FDA Form 483 Warning Letter, Now What?
Remember the last time you were pulled over by the police? Maybe you had a taillight out. Perhaps you were speeding. You may have believed that you had done nothing wrong. Regardless of the specific...

Quality & Compliance
Demystifying CAPA Management: Overcoming Challenges in the Fast-Paced World of GMP
This article has been updated since its original publication date. Navigating the complexities of Corrective Action / Preventive Action (CAPA) in the drug and medical device industries often poses a...

Clinical Research Solutions
Clinical Trial Good Clinical Practice (GCP) Audits – Are you ready?
Good clinical practice (GCP) is an international ethical analysis and scientific quality standard for designing, conducting, and auditing clinical trials that involve the participation of human...

Quality & Compliance
The Importance of Responding to FDA 483 Observations
This article has been updated since its original publication date. The FDA has an established policy that allows companies 15 days to respond in writing to the FDA after issuance of a 483...

Quality & Compliance
FDA Form 483: Common Pitfalls You Can Avoid
This article has been updated since its original publication date. FDA Form 483 requires a written response in which you must make it clear that you are taking the observations, and your...
Clinical Research Solutions
Changes to the Animal Welfare Act Affecting Animal Research Facilities
Does your organization conduct or outsource testing to an Animal Research Facility? If so, are you aware of the changes that have been implemented to the AWA (Animal Welfare Act) by the Animal and...

Clinical Research Solutions
Be Careful What You Ask For (Prior to Consent)
According to FDA’s clinical trial regulations (21 CFR 50.20, 312.60 and 812.100), clinical investigators are responsible for protecting the rights, safety, and welfare of subjects during a clinical...

Clinical Research Solutions
ProPharma Group's Dr David Crome to Act as Compliance Monitor to the MHRA
From April 2022, the MHRA has been developing a pilot programme for GMP and GDP remediation supervision by eligible consultants acting as Compliance Monitors (CM) on behalf of companies that have...