
Quality & Compliance
Navigating EU GMP Compliance: A Consultant's Guide to Smooth Sailing
Hello, fellow pharma enthusiasts! In the fast-paced world of pharmaceuticals, ensuring Good Manufacturing Practice (GMP) compliance is essential to maintaining product quality and safety. The...

Quality & Compliance
Staying GMP Compliant: A Consultant's Guide to Compliance Bliss
Hello, dear readers and fellow compliance enthusiasts! Welcome to our journey through the labyrinth of Good Manufacturing Practices (GMP) compliance. As a consulting company that provides audit...

7 Critical Factors for Successful Selection of CDMO for Cell and Gene Therapy Manufacturing
Developing, optimizing, and manufacturing Advanced Therapy Medicinal Products (ATMP's), such as Cell and Gene Therapy (CAGT) products is extremely complex. The choice of a reliable Contract...
Quality & Compliance
Prepare for Your Next Audit: A 5-Point GMP Checklist
Ensuring you have full control over your processes, facility, and quality management system (QMS), and ultimately your final product quality, is a demanding and important task. An inability to do so...

Why the FDA Should Never Be Your First Inspection
You can expect several FDA audits throughout your drug development program The Agency’s goal is to protect the public from unsafe products, and one of the best ways to accomplish that goal is by...
Regulatory Sciences
Outsourcing Functions Doesn't Mean You've Outsourced Compliance Obligations
Over the past 20 years, the traditional approach to drug development has expanded to include the outsourcing of a range of testing and manufacturing functions. As a part of their long-term strategic...
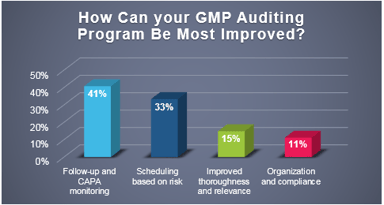
Industry Poll: How Can Your GMP Auditing Program Be Most Improved
Recently, ProPharma conducted a poll to quality professionals across the country to understand the challenges that FDA regulated companies face in managing their GMP auditing programs. As depicted in...
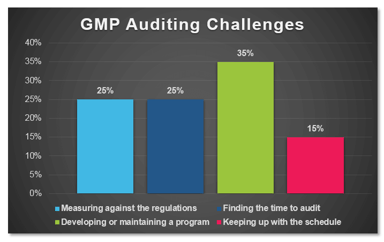
Industry Poll: What are the Leading Challenges with GMP Auditing?
In a recent poll conducted by ProPharma Group, the question “What is your biggest GMP auditing challenge?” was posed to Quality professionals in the drug manufacturing industry. The following graph...
Quality & Compliance
Clinical Quality Systems & the Outsourced Model
The landscape of clinical trials is evolving. The changes that are happening are due to the increased number of FDA-regulated trials, as well as a rise in the complexity of clinical protocols. As...
Quality & Compliance
#7: Quality Systems Approach to Pharmaceutical CGMP Regulations
In August 2002, the FDA announced the Pharmaceutical CGMPs for the 21st Century Initiative, which explained FDA’s intent of integrating quality systems and risk management approaches, and had a goal...
Regulatory Sciences
FDA Addresses Extrapolating Adult Data for Pediatric Use
In 2004, the FDA issued a guidance document, entitled “Premarket Assessment of Pediatric Medical Devices.” The guidance stated that, when consistent with scientific principles, data can be...
Everything you to need to know about Audit Trails
In today’s validated lab environment, knowing the importance of an audit trail in computerized laboratory systems is just one of the integral qualification tasks that the ProPharma’s Computer System...
Supplier Qualification in the Pharmaceutical Supply Chain
The Food and Drug Administration Safety and Innovation Act (FDASIA), was signed into law on July 9, 2012. This law significantly expanded the FDA’s authority and strengthened its ability to safeguard...
Regulatory Sciences
The FDA’s Enforcement of Section 503B of the Federal Food, Drug, and Cosmetic Act
The FDA publishes weekly enforcement reports highlighting drugs that have been recalled during the previous week. Over the past several months these reports have been littered with hundreds of...