
Quality & Compliance
Staying GMP Compliant: A Consultant's Guide to Compliance Bliss
Hello, dear readers and fellow compliance enthusiasts! Welcome to our journey through the labyrinth of Good Manufacturing Practices (GMP) compliance. As a consulting company that provides audit...

Quality & Compliance
Compliance Auditing: A Peek into Common Critical Findings
Compliance auditing is a crucial process in the pharmaceutical industry, that helps ensure the safety, effectiveness, and high quality of pharmaceutical products and medical devices. Compliance...

Quality & Compliance
The Importance of Responding to FDA 483 Observations
This article has been updated since its original publication date. The FDA has an established policy that allows companies 15 days to respond in writing to the FDA after issuance of a 483...
Quality & Compliance
Top Tips for a Successful Virtual Audit
The COVID-19 pandemic forced countless companies to change the way they do business in order to continue operating while protecting employees and upholding their social responsibility to the...
Quality & Compliance
Embracing Risk Management Principles: As Easy as One, Two, Three
In the pharmaceutical industry today, there are strict guidelines to adhere to throughout the product lifecycle. Some of them, like risk management, seem more complex and harder to adhere to than...
Quality & Compliance
Get the Most Out of Your GMP Effectiveness Checks
We work in a highly regulated industry. Whether you are associated with the manufacturing of a drug, a biologic, or a device, you understand the importance of those regulations on the safety and...

Deviations: Beyond the Basics
There are plenty of guidelines and instructions on implementing a deviation system in a pharmaceutical/medical device company. However, there is a big difference between theory and practice when it...
Quality & Compliance
FDA's Top 483 Observations for 2018: A Reflection of Industry’s Compliance
At the beginning of each federal fiscal year, the US FDA posts the previous year's Form 483 observation metrics issued by each product center. Inspections ending between 10/1/2017 and 9/30/2018, for...

Health Apps and the Requirements Imposed By the Law
If you check Apple’s App Store or Google’s Play Store you will find an overwhelming list of health and fitness apps. This list only gets longer, if you include the number of people who use these...
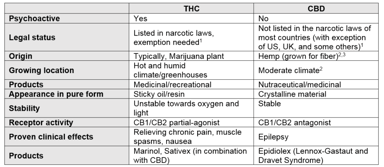
7 Things to Consider in Medicinal Cannabis Development
Mention the word cannabis and the confusion starts; legal or illegal, nutraceutical or medicinal product, psychoactive or non-psychoactive, clinically significant or not. At the same time, the...
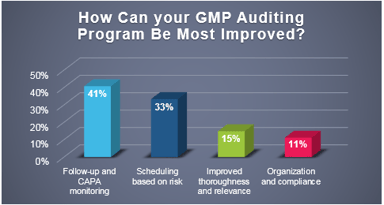
Industry Poll: How Can Your GMP Auditing Program Be Most Improved
Recently, ProPharma conducted a poll to quality professionals across the country to understand the challenges that FDA regulated companies face in managing their GMP auditing programs. As depicted in...
Outdated Facilities – Bring Back the ‘c’ in cGMP
New rules, old facilities. How do these two meet? Is it a big black hole or is there light at the end of the tunnel? When you work in an older facility, you are probably acquainted with one-liners...
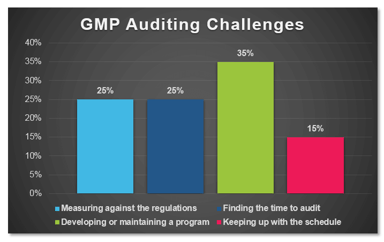
Industry Poll: What are the Leading Challenges with GMP Auditing?
In a recent poll conducted by ProPharma Group, the question “What is your biggest GMP auditing challenge?” was posed to Quality professionals in the drug manufacturing industry. The following graph...
Quality & Compliance
Implementing a Risk-Based Supplier Management Program
According to recent FDA updates on the implementation of the Safety and Innovation Act (FDASIA), nearly 40 percent of finished drugs are being imported, and nearly 80 percent of active ingredients,...
FDA's Top 483 Observations for 2017: A Reflection of Industry's Compliance
At the beginning of each federal fiscal year, the US FDA posts the previous year’s Form 483 observation metrics issued by each product center. I find that reviewing these metrics provides a valuable...
Quality & Compliance
How to Write an Effective Quality Investigation Report
In 2016, the FDA issued hundreds of 483 observations across the Drug and Device industries for failing to thoroughly review or investigate issues. This topic consistently hits the top five most...