
Regulatory Sciences
CMC Expectations During Drug Substance Transfer from Ex-US Manufacturers
Regulatory Drivers for US-based API Manufacturers Recent shifts in US tariff policies have introduced new pressure points in the global pharmaceutical supply chain, particularly for manufacturers...

Regulatory Sciences
Regulatory Chemistry, Manufacturing, and Controls (CMC): What to Expect During Drug Development
The key to successful drug development in the US is directional and focused navigation of FDA’s Investigational New Drug (IND) process. The Chemistry, Manufacturing, and Controls (CMC) section is a...

Regulatory Sciences
Highlights from FDA's Analytical Test Method Validation Guidance
Recently, the FDA updated a long-standing, decades old guidance on analytical test method validation based on revisions of the ICH Q2(R2) guidelines. Traditional test method validation requirements...
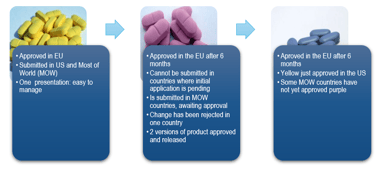
CMC Regulatory Dossier Compliance: A GMP Requirement
Maintaining compliance in the dynamic regulatory Chemistry, Manufacturing and Controls (CMC field can be quite a challenge. A CMC regulatory dossier compliance assessment is a critical component and...
Everything you to need to know about Audit Trails
In today’s validated lab environment, knowing the importance of an audit trail in computerized laboratory systems is just one of the integral qualification tasks that the ProPharma’s Computer System...