
Regulatory Sciences
The Moving Regulatory Landscape for Gene Therapy Trials in EU: Part 2
Change in the Submission of the Summary Notification Information Format The Summary Notification Information Format Form As of January 31, 2023, Sponsors are required to submit a Clinical Trial...

Regulatory Sciences
The Moving Regulatory Landscape for Gene Therapy Trials in EU: Part 1
The Benefit of the Common Application Form for GMO Applications GMO Application Not Included in Clinical Trial Regulation With the transition period for the Clinical Trial Regulation No 536/2014...

Quality & Compliance
5 Key Challenges in the Development of Gene Therapies
Over three decades of gene therapy (GT) clinical trials have yielded many innovative and promising treatments for pernicious genetic disorders but industry has shown slow development and dashed...

Medical Information
The Role of Patient Support Services in the Evolving World of Cell & Gene Therapies (CAGTs)
Cell and Gene Therapies (CAGTs) represent a transformative step in medicine, offering unprecedented potential to treat and potentially cure diseases once deemed untreatable. However, the path to...

Quality & Compliance
How to Decentralize Cell and Gene Therapy Treatments
The Challenge: CAGT Treatments Require Time and Lack Availability Persons diagnosed with life threatening diseases which are now treatable with cell and gene therapies (CAGT) often needed to wait for...

Quality & Compliance
FDA Publishes Guidance for CAR T Products
In January 2024, the FDA Center for Biologics Evaluation and Research (CBER) Office of Therapeutic Products issued a guidance document- Considerations for the Development of Chimeric Antigen Receptor...

Clinical Research Solutions
Decentralization of Cell and Gene Therapy
Patients typically go through the same thought process when they have been diagnosed. After the relief of knowing that their ailment was correctly diagnosed, most patients want to know the path back...
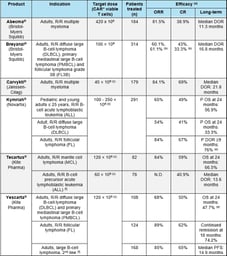
Clinical Research Solutions
CAR-T Cells: Challenges, Lessons Learned, and Guidance for the Clinical Development
It comes as no surprise to any pharmaceutical or biotech company that planning the clinical development of CAR-T cells is an extremely challenging endeavor: high efficacy is expected in each targeted...
Clinical Research Solutions
Phase Appropriate Controls and GMPs in Cell and Gene Therapy
How much is too little versus too much when developing quality systems and controls for investigational cell and gene therapies? Because these therapies are being administered to patients during all...
Regulatory Sciences
A Guide to Understanding Long Term Follow-Up for Gene Therapy Clinical Trials
When conducting a clinical trial, there are many aspects sponsors need to be aware of with regards to clinical safety, product efficacy, and the ability to bring treatments through the multiple...