Regulatory Sciences
FDA Accelerated Approval Pathway: A Potential Missed Opportunity for Sponsors
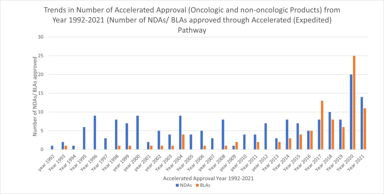
The accelerated approval provisions of FDASIA in section 506(c) of the FD&C Act provide that FDA may grant accelerated approval to: . . . a product for a serious or life-threatening disease or...
Battle of the Regulators: Comparing FDA’s Accelerated Approval and EMA’s Conditional Marketing Authorisation
The Food and Drug Administration (FDA) and the European Medicines Agency (EMA) offer various pathways that can expedite the development and review of new therapies to treat serious or...