Navigating FDA Layoffs: How Policy Layoffs May Impact Generic Drug Development
Layoffs at the Office of Generic Drug Policy Could Slow Development—but Strategic Guidance Can Help Sponsors Stay on Track

Clinical Research Solutions
ProPharma Group has launched a “Meet the Expert” series introducing you to our experts from around the world. This series will help you get to know who we are, and how our colleagues work to support...
Clinical Research Solutions
Big data is hitting us from all angles, and life science industries are not being left out. Why? Your life depends on it, literally. Life sciences generate lots of large and complex data every single...

Clinical Research Solutions
FDA Approval Process Overview The Food and Drug Administration (FDA), as part of the United States (US) Department of Health and Human Services, is the regulatory agency responsible for the review,...

Clinical Research Solutions
Adverse drug reactions (ADRs) are a significant cause of deaths and emergency hospital visits. The good news is that monitoring and understanding ADRs can help minimize and even prevent such events...
Clinical Research Solutions
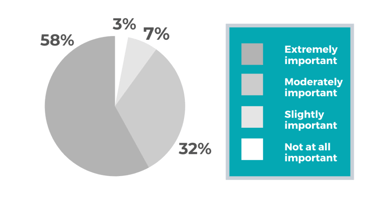
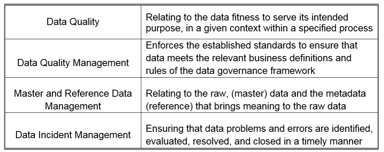
The attention of regulatory agencies continues to focus on data integrity, as observed by the increase of FDA observations over the course of the last few years. Having a proper data lifecycle / data...

Clinical Research Solutions
Over-the-counter (OTC) drugs are defined by the FDA as drugs that are safe and effective for use by the general public without seeking treatment by a health professional. Currently, there are more...
Clinical Research Solutions
Get the Most Out of Your GMP Effectiveness Checks: We work in a highly regulated industry. Whether you are associated with the manufacturing of a drug, a biologic, or a device, you understand the...
Clinical Research Solutions
We are living through a medical revolution. Advances in gene therapy, cell‑based therapies and tissue engineering offer real hope for patients with a range of debilitating diseases. The FDA, EMA and...