Regulatory Sciences
FDA Thinking on Biosimilar Immunogenicity Issues - A Case Study Based on the Enoxaparin ANDA Approval
Following extensive marketing of Lovenox® (enoxaparin sodium), a low-molecular weight heparin, a number of biosimilar versions of enoxaparin and one ANDA-approved formulation (enoxaparin sodium,...
Quality & Compliance
GAMP® 5 Basis for Quality System Compliance
Next week I’ll be in San Francisco conducting an ISPE training class on Risk Based Approach to GxP Process Control Systems. Attendees will include employees from a variety of regulated life cycle...
Quality & Compliance
Process Validation: Gate Eight of the Nine Gate Transfer Process
Today we have the next phase of the Nine Gate Transfer Process: Gate 8 Process Validation. To review the previous steps before continuing, examine Gates 1-7 here: Gate 1: Assessment Gate 2:...
Cleaning Disaster or True Worst Case?
How robust are your cleaning validation measures? Could your storage procedures withstand the impacts of a fire within your facility, or would such a disaster set back your production time...

Improving Computerized System Quality Through Design Verification
Unverified Design – An Example For those of us who travel routinely, one of the most sought-after treasures in the typical airport terminal is an electrical outlet. With our dependency on mobile...
Quality & Compliance
Evaluating the Effectiveness of a Corporate Compliance Program: How Does Your Program Measure Up?
At the recent Pharmaceutical Compliance Congress (PCC), Zane Memeger, U.S. Attorney for the Eastern District of Pennsylvania stated that one of the key questions that a company should ask about its...
Regulatory Sciences
What Can Testosterone Gels Teach Us About Abuse-Deterrent Opioids?
For most drugs, the process of developing and obtaining FDA approval of a generic version is simple and well-defined: sponsors must only prove that their product is pharmaceutically equivalent and...
Quality & Compliance
Ready for cGMP Validation: Gate Seven of the Nine Gate Transfer Process
Today, Bob Beall is back with the next gate in the Nine Gate Transfer Process. If you have missed any of the previous steps in this process, you can review them here: Gate 1: Assessment Gate 2:...
Rethinking the OTC Monograph System
When the OTC drug review was undertaken in the 1970s, it was deemed the best solution available at the time for efficiently assessing the safety and efficacy of the countless drug products being sold...
Quality & Compliance
To Requalify or to Continuously Monitor, That is the Question
Your manufacturing equipment and processes have been established, qualified and validated; all you have to do now is rake in the profits right? Think again, the process of maintaining the validated...
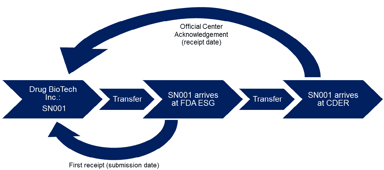
Words, Words, Words: The Importance of Diction in Regulatory Submissions
In continuation of its series of guidances on electronic submissions, FDA recently released a guidance for receipt dates of electronic submissions which emphasizes one important fact: word choice...
Computer System Validation: Resolutions for the New Year
How are you doing with those New Years’ resolutions? Whether or not you’re a resolution maker (or breaker), you can use this time of year to take stock of where things stand, including your validated...
Impact of Wavelength Accuracy and Precision on Spectrophotometric Measurements
A while back I was involved in a Quality Assurance project reviewing calibration specifications for analytical instrumentation. The client had implemented a new requirement that all calibrated...
Quality & Compliance
Formulation and Process Development: Gate 6 of the Nine Gate Transfer Process for Moving Production
Today, we are continuing our blog series on the Nine Gate Transfer Process for Moving Production from Site to Site, with Gate 6: Formulation and Process Development. If you have missed any previous...
Regulatory Sciences
FDA Revisits the Topic of INDs for Dietary Supplements
One of the fundamental disconnects in the dietary supplement world is that despite the fact that manufacturers of dietary supplements are not allowed to make claims regarding effects on diseases,...






