Outdated Facilities – Bring Back the ‘c’ in cGMP
New rules, old facilities. How do these two meet? Is it a big black hole or is there light at the end of the tunnel? When you work in an older facility, you are probably acquainted with one-liners...

Clinical Research Solutions
CBER Provides Sponsors with Policies and Procedures Regarding INTERACT Meetings
On Monday, October 1, FDA’s Center for Biologics Evaluation and Research (CBER) issued a document outlining the policies and procedures for scheduling and conducting INitial Targeted Engagement for...

Good Review Management Principles & Practices, Part One: Fundamental Values
On Tuesday, September 25th, the FDA published a draft guidance containing recommendations on good review management principles and practices (GRMPs) for new drug applications (NDAs), Biologics...
Regulatory Sciences
FDA Releases Draft Guidance on Benefit-Risk Determinations for Devices
On Thursday, September 6th, the FDA released a new draft guidance regarding benefit-risk determinations in medical device premarket approval applications (PMAs), De Novo requests, and humanitarian...
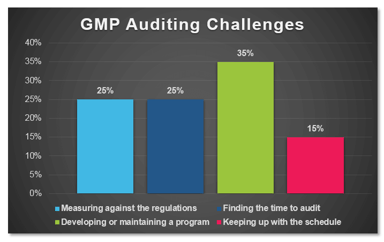
Industry Poll: What are the Leading Challenges with GMP Auditing?
In a recent poll conducted by ProPharma Group, the question “What is your biggest GMP auditing challenge?” was posed to Quality professionals in the drug manufacturing industry. The following graph...
Clinical Research Solutions
Generic Failure: Why so Few ANDAs Are Accepted by FDA on the First Pass
Generic drugs are immensely important to the U.S. healthcare system. These drugs account for 89% of the prescriptions dispensed in the United States. And, over the last decade, generic drugs have...

FDA Prescription Drug User Fee Rates: Fiscal Year 2019
On Wednesday, August 1st, FDA released a notice with updated prescription drug user fee rates for fiscal year 2019. Prescription Drug Application Fees According to the Food, Drug, & Cosmetic (FD&C)...
Quality & Compliance
Implementing a Risk-Based Supplier Management Program
According to recent FDA updates on the implementation of the Safety and Innovation Act (FDASIA), nearly 40 percent of finished drugs are being imported, and nearly 80 percent of active ingredients,...
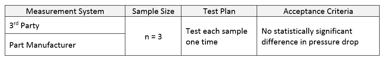
The Power to See Differences that Matter
Data collection and analysis is expensive and has the potential to compromise a company’s hard-won compliance position. It is therefore critical that technical leaders follow a systematic and proven...
Pharmacovigilance
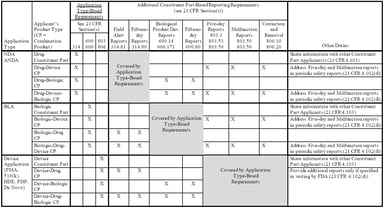
Understanding the New Combination Product PMSR Guidance Documents and Impact on Industry
On March 20, 2018, the US Food and Drug Administration (FDA) released two new guidance documents to help companies comply with the December 20, 2016 final rule establishing postmarketing safety...
MassBio Annual Meeting: Event Highlights
MassBio is a non-profit organization that represents and provides support for the life sciences supercluster in Massachusetts. MassBio is committed to growing the industry, adding value to...
Regulatory Sciences
FDA Steps up its Game on Generic Drugs: The Story Behind the Recent Focus on Generic Products
Throughout 2017, the FDA focused its attention on the regulation of generic drug products. In 2015, the Agency issued only two generic-related guidance documents. In 2016, there were seven. In 2017,...
Clinical Research Solutions
Finding a Roadmap to Approval - Hint: You Can't Pick One Up at the FDA
Earning FDA approval for your drug program is a journey. A misstep at any point of that journey could jeopardize your chance at getting your drug approved. That’s why it’s so important to have a...
FDA's Top 483 Observations for 2017: A Reflection of Industry's Compliance
At the beginning of each federal fiscal year, the US FDA posts the previous year’s Form 483 observation metrics issued by each product center. I find that reviewing these metrics provides a valuable...
Quality & Compliance
Transformational Leadership: Part II
Last week, I introduced a two-part series on transformation leadership. We defined transformational leadership, explored the concept, and discussed the benefits of adopting this approach. Well, now...






