October 19, 2022
October 19, 2022

An unlikely beacon of hope from the otherwise disastrous Covid pandemic, may come in the form of renewed attention towards approaches to vaccine development.
The development of vaccines has been shown to be both lucrative and crucially important for disease control, both in humans and animals. The enormous variability in disease-causing agents and their variants requires a large repertoire of approaches to effectively present neutralizing epitopes to the immune system.
Traditional vaccination strategies, utilizing inactivated viral, bacterial, and toxoid preparations or live-attenuated strains, have successfully controlled diseases such as tetanus and polio. Safer formulations composed of purified polysaccharides, recombinant proteins or RNA have yielded effective vaccines against pathogens such as Streptococcus pneumoniae, Hepatitis B virus and coronavirus. In addition to infectious diseases, vaccines or vaccination approaches may also be used to treat for example oncological diseases.
Novel adjuvants; nanotechnology; new delivery systems; alternative delivery routes and vaccine strategies utilizing a multi-antigen expression platform facilitate new vaccination approaches.
The development of a novel vaccine is a complex and lengthy process that generally takes 10 to 15 years.
Usually after seeking scientific advice about acceptable potency and clinical parameters and after approval of an IND and/or an IMPD, the potential vaccine may proceed through three phases of testing in humans.
Successful completion of clinical studies will demonstrate that the candidate vaccine is safe and effective and a Biologics License Application (BLA) or a Marketing Authorization Application (MAA) is submitted to applicable authorities to obtain a license. This entire process could take up to 2 years.
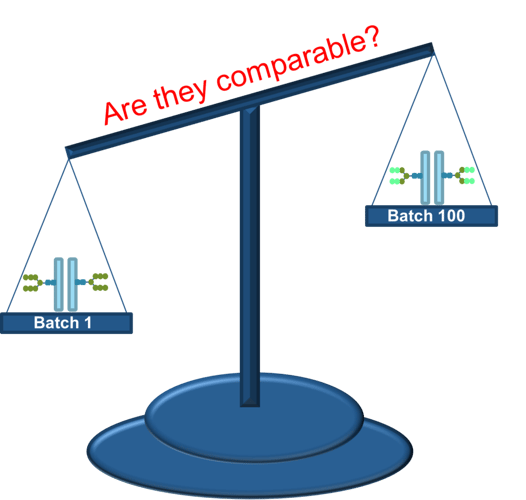
Vaccines are developed to present disease related epitopes to responsive immune cells that will initiate a protective/curative immune response. To achieve this many choices are to be made based on the understanding of the disease and its relevant epitopes, and subsequently based on the risks anticipated during manufacturing and during use in humans (or animals). As the quality of a biological substance may--to a large extent--rely on the validated capacity of the production process to yield a product of consistent quality, the careful choice of the vaccine approach becomes even more important.
Traditional vaccine preparations, utilizing inactivated viral, bacterial, and toxoid preparations or live-attenuated strains may involve less complex and low-cost manufacturing processes, are usually less safe, but may offer a strong and broad immune response. Safer formulations composed of purified polysaccharides, recombinant proteins or RNA may involve more complex and higher cost manufacturing processes. As these preparations in general offer a relatively limited set of epitopes, the obtained immune response may be less strong and less broad.
Freedom from adventitious agents: virus, TSE, and microorganisms, which could be present as a consequence of the biological production methods and biological starting materials. Next to quality control on raw materials, virus testing (and virus removal capacity), bioburden/sterility testing and aseptic control validation, an overall risk assessment for adventitious agents is required.
The short answer is yes.
With the establishment of new vaccine approaches including adjuvants; nanotechnology; new delivery systems; alternative delivery routes and multi-antigen platforms, it is expected that existing and new future diseases can be approached more effectively. Nevertheless, the development of vaccines still requires careful planning of the following aspects:
ProPharma Group can assist you with many different types of vaccine projects and our experienced regulatory sciences consultants can offer support and guidance at each phase of development and throughout your product’s lifecycle.
We can support and author:
We can support, consult, and advise on:
Interested in learning more? Contact us today to find out how we can help with your global regulatory needs.
TAGS: Regulatory Sciences

August 21, 2023
COVID-19 has presented humankind with the challenges expected from a pandemic with over 700 million cases and almost 7 million deaths1. While the mRNA technology was not widely known in the news, it...
July 5, 2022
News 01/07/2022 On 30 June, regulators from around the world discussed emerging evidence to support adaptation of COVID-19 vaccines as the SARS-COV-2 virus continues to evolve during a workshop...
August 12, 2020
Preventive vaccines are a special kind of medicinal product, as they are administered before a disease instead of during. You are probably familiar with preventive vaccines from your own or your...