May 18, 2023

May 18, 2023

Our "Meet the Expert" series introduces you to our team of experts around the world. This "behind the curtain" view will help you get to know who we are on a professional and personal level, and highlight how our colleagues work together on our higher purpose to improve patient health and safety throughout the complete product lifecycle.
I work as the Director for the Functional Services Provider (FSP) Team within Clinical Operations. ProPharma's FSP model is an outsourcing solution that focuses on building and managing expert functional teams to execute clinical studies. Sponsors can boost outcomes in specialized services or niche markets by utilizing our worldwide resource access and our expertise in clinical research. By offering a collaborative solution to our clients, we deliver an integrated clinical trial approach with shared industry experience and expertise that improves patient health and safety.
The FSP model provides exposure to diverse perspectives and approaches while contributing to the advancement of medical science. We have the opportunity to gain valuable experience and exposure to a variety of study designs and therapeutic areas from our clients. We can use this to our advantage by developing a broader perspective on clinical trial management while building a versatile skill set that can be applied to different settings. Additionally, since many of the FSP programs are set within the client's environment, we can "flex" our skills in adaptability, flexibility, and communication- which are critical skills in a complex and dynamic industry like clinical trials.
With the current economic landscape, several sponsors are struggling to control the expenses associated with clinical trials. Our bespoke approach within a competitive market involves cost management strategies that center on supplying value to sponsors. We offer solutions that balance the need for high-caliber services with cost efficiency which allow companies to grow in infrastructure by managing HR costs and risks. One of the greatest benefits of the FSP model is that it allows for flexible resourcing during the ebbs and flows of clinical trials by minimizing the financial burden between projects and allowing companies the ability to not only ramp up/down but also move resources internally.
Our work at ProPharma provides sense of purpose and satisfaction by contributing to the development of new treatments and therapies that can have a positive impact on patient health and well-being. Our company culture fosters a sense of pride and commitment to the work being done in each clinical trial we support. I am proud to work for an organization that values collaboration, works towards excellence and promotes innovation!
TAGS: Meet the Expert
November 11, 2019
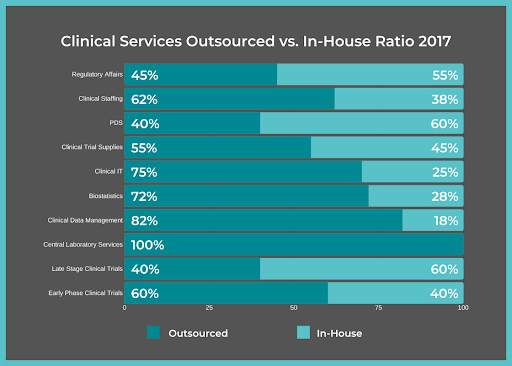
When pharmaceutical companies launch a clinical trial or reach a certain phase of Clinical Development, with only the support of their in-house employees, the additional workload often becomes too...

July 15, 2024
The term Functional Service Provider (FSP) concept is highly regarded in the field of clinical research. With drug discovery and clinical trials becoming more complex, pharmaceutical firms and...