When applied rigorously and comprehensively, lean principles can reduce organizational costs while increasing productivity and strengthening the overall quality of submissions
What is “Lean” as it Applies to Medical Writing?
- Lean medical writing emphasizes the use of precision in organizing large volumes of data, with a focus on targeted data analysis and presentation.
- Central themes are streamlined content at the document and overall submission level, the avoidance of repetitive, unnecessary text, and the use of hyperlinking to other documents rather than a repeat of information across documents.
Why Lean?
- Developed as a means of reducing country and regional differences in technical requirements to ensure availability and competitive cost structures for bringing new medicines to market.
- Promotes the international movement of pharmaceuticals that are safe, effective, and of high quality.
- Conforms to standards in conducting human clinical trials and in data collection to align with international standards.
General Principles
- Use straightforward language, short sentences, bullet points, and summary highlights of tabulated data.
- Create a shared library of documents, guidelines, key messaging tools, and templates at product level, project level, or company level for reference to ensure all stakeholders are aligned.
- Created a detailed guideline of lean principles as they apply at the document level. For example, Clinical Study Reports (CSRs), Clinical Overviews and Summaries, etc.
- Remove redundant data and information; for example, in a CSR, hyperlinking back to the appropriate protocol section rather than repeating information already in the protocol.
- Streamlining the processes and guidelines for exploratory analysis; avoiding extra analyses and data published broken into multiple small pieces or multiple analyses.
Aspects of Lean for Writing a Clinical Study Report
- Historically, CSR content and format have varied across sponsors, adding unnecessary complexity for regulatory reviewers.
- As a result of the need to streamline CSRs, the CORE (Clarity and Openness in Reporting: E3-based) Reference was released May 2016 by the European Medical Writers Association (EMWA) and the American Medical Writers Association (AMWA).
- CORE is an open-access user’s guide to support the authoring of CSRs; the guidance not only helps ensure medical writers are following comprehensive lean writing principles, but also facilitates the development/creation of streamlined templates at the organizational level.
- CORE Reference presents focused CSR structure and content addressing current regulatory guidances, including public disclosures.
- As per the underlying ICH E3 document, CORE Reference is a reference tool companion to ICH E3, and not a template. The content suggestions are arranged in sections, which are mapped to the principal regulatory guidance, ICH E3, using the CORE Reference mapping tool, allowing users to contextualize CORE Reference within ICH E3.
- CORE Reference is the only resource that identifies all current places in an ICH E3-compliant CSR where disclosure considerations apply.
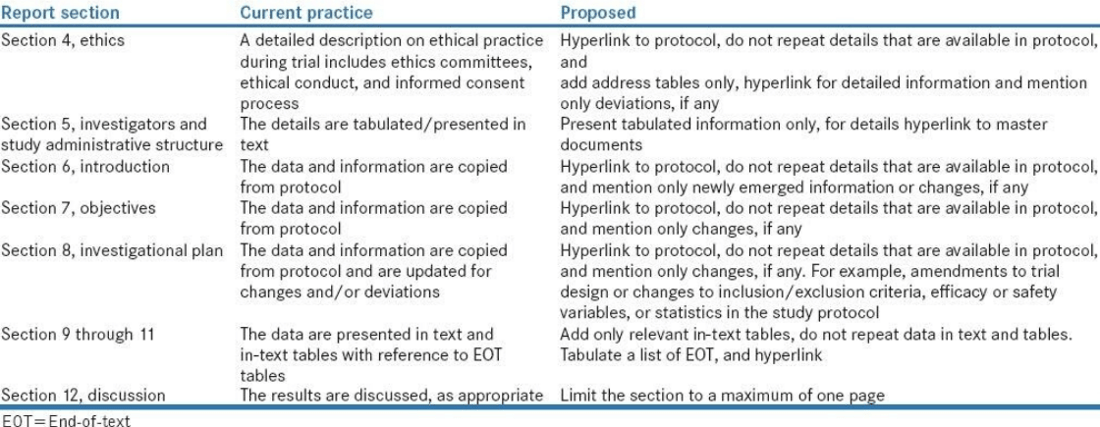
Refer to Table 1 for an example of the Lean process as it applies to CSR writing.
Table 1 Lean Example for CSR Writing
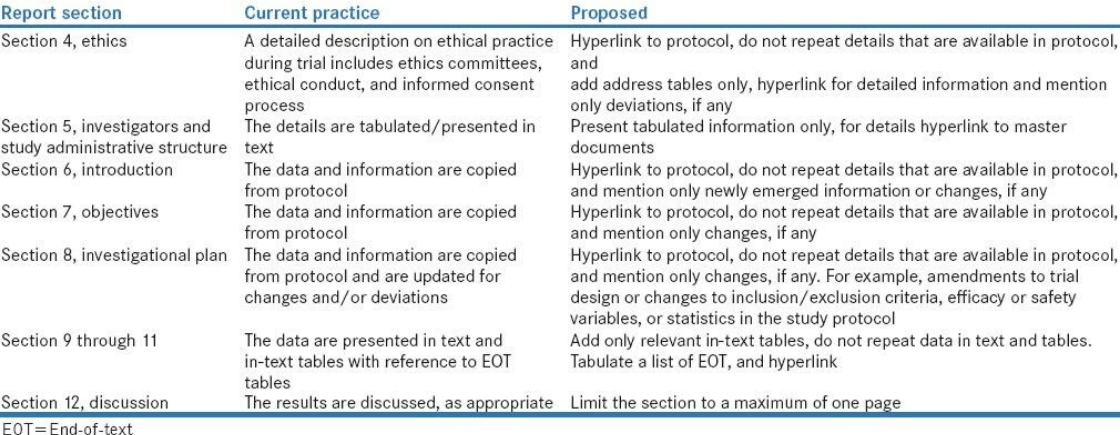
Source: Payal Bhardwaj, Sudip Sinha, and Raj Kumar Yadav. Perspect Clin Res. 2017 Jul-Sep; 8(3): 113–117.
Key Challenges
- Implementation – ensuring an appropriate suite of document templates, guidance, and training.
- Communication – building a comprehensive communication platform across the organization to facilitate better understanding of individual roles and responsibilities as applied to the lean process.
- Alignment – management structure to ensure all functional areas of an organization are trained and in alignment with lean principles.