October 15, 2019
October 15, 2019

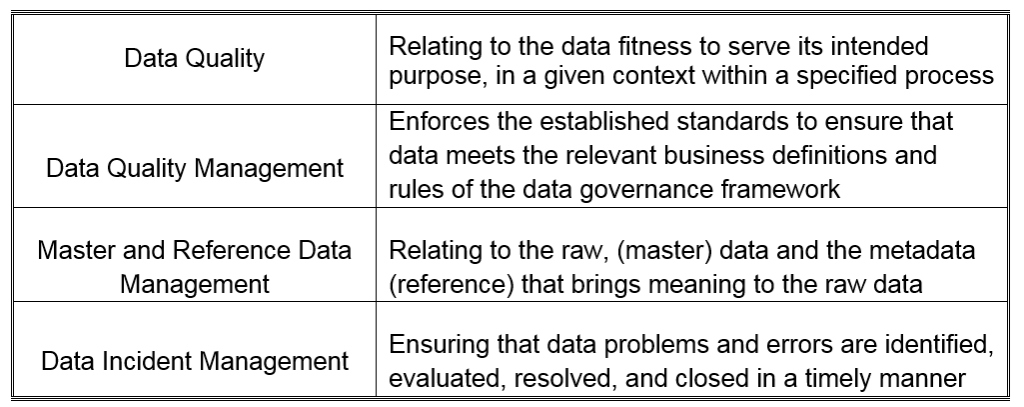
The attention of regulatory agencies continues to focus on data integrity, as observed by the increase of FDA observations over the course of the last few years. Having a proper data lifecycle / data management program in place, requires a mature governance framework to be established around the following;
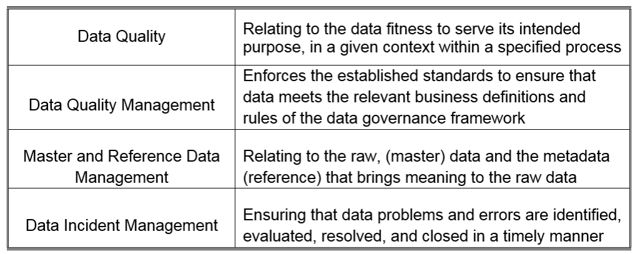
As noted in the Record and Data Integrity Guide published by ISPE® (International Society for Pharmaceutical Engineering), elements of data governance are closely related to regulatory requirements. Data governance activities should be an integral consideration to a Quality Management System, (QMS). A specific corporate data integrity program may be necessary to address any gaps in a regulated company’s existing Quality Management System.¹
But let’s say you already have a robust QMS in place, whether it be automated or well written manual processes, how do you know whether it has matured to the degree necessary to satisfy regulatory agencies’ expectations? Do your policies truly reflect your current business needs? Do you have a monitoring program in place that results in continuous improvement in your processes? You see, just as important as having a well-documented data governance framework is the need to be able to quantify how effective your ongoing efforts are. Without quantifying your program, you will fall short in being able to measure your program’s maturity level. For example, you need to know what level the characterization of your data lifecycle is, and potentially what else needs to be tasked to get your processes to the standards that are expected.
What is a Data Integrity Maturity Level Characterization?
Gaining the understanding of your current state of maturity can be accomplished with the use of a maturity characterization model. Simply put, you establish the characteristics at each level and what is needed to advance to a higher degree of maturity. This can also assist in improving existing work practices. In our example, the effectiveness ranking spans across five, (5) levels;
The scale for this assessment was 1 to 3;
A recent data integrity assessment performed at a regulated manufacturing site focused on the maturity characterization of their data lifecycle. Seven (7) critical elements were assessed for their primary manufacturing system;
This example given in figure 1 is a sample of the data integrity assessment results;
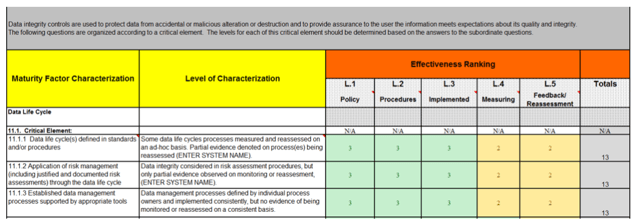 Figure 1
Figure 1
Creating and utilizing such a model creates the needed visual to help organizations reached the next level of effectiveness. By implementing and maintaining this type of program you are demonstrating a quantifiable means of improving your effectiveness in data management. ProPharma Group has the experts to perform data integrity assessments across all aspects of data integrity; Governance, Strategic Planning, Data Lifecycle, and Data Lifecycle supporting processes. Contact us to learn more how our prescribed two-day data integrity assessments can benefit your organization.
¹ISPE GAMP® Records and Integrity Guide

June 5, 2013
ISO 13485 is a regulatory standard whose focus is meeting customer requirements, including regulatory requirements, and maintaining the effectiveness of the Quality Management System (QMS). Section 5...
September 29, 2021
Are you always ready to be inspected for Data Integrity (DI) activities in your facility? Are compliance and data integrity aspects implemented in your organization’s QMS? Are the systems in your GxP...