January 21, 2016
January 21, 2016
Over the past few years, we have seen a drastic upsurge in the amount of FDA activity involving Indian Active Pharmaceutical Ingredient (API) manufacturers.
Indian-based pharmaceutical manufacturers are a leading source of API and generic drug products in the United States. Furthermore, Indian pharmaceutical manufacturers are responsible for the production of about 15% of all generic products in the US; and, India is home to more than 550 FDA registered manufacturing sites. These are both indications of India’s widespread impact on the US pharmaceutical industry. With this increasingly prominent presence, The FDA feels that it is extremely important for the Agency’s efforts in India to be equally as robust in order to assure that the products being exported meet US safety and efficacy standards.
The Central Drugs Standard Control Organization (CDSCO) is India’s “Central Authority,” and this agency is responsible for the regulation of pharmaceutical products within the country. According to CDSCO’s website, it is “responsible for approval of New Drugs, Clinical Trials in the country, laying down the standards for Drugs, control over the quality of imported Drugs, coordination of the activities of State Drug Control Organizations.”
Although CDSCO claims to inspect the manufacturing facilities’ of local and multinational companies, the reports from these inspections are not made publicly available. Additionally, CDSCO representatives’ attendance at FDA’s inspections of Indian manufacturers is extremely rare.
Data integrity “refers to the accuracy and consistency of data generated during GxP.” The integrity of the data collected by pharmaceutical manufacturers is essential in order to confirm that the products in question are high-quality and safe for human use. Issues with data integrity pose a huge risk to consumers, and the FDA works very hard to ensure that manufacturers comply with all Good Manufacturing Practices (GMPs).
According to the FDA, one of the biggest issues facing the Indian pharmaceutical manufacturers involves following the Agency’s data integrity guidelines. Issues such as workforce shortages, absence of understanding of GMPs, or simply compromising acceptable quality levels to meet production goals, have caused the Agency to feel that data integrity is an issue being faced by some manufacturers in India.
Data integrity is considered to be the foundation upon which decisions on quality, efficacy, and safety are made in the US. Since 2010, the FDA has sent at least 50 Form 483s (the precursor to a Warning Letter), and issued nearly ten Warning Letters to Indian manufacturing companies alleging improper data controls.
TAGS: Agency Alerts
August 24, 2016
Last week the FDA released an astonishing 23 warning letters along with an import alert to a number of companies located across the globe. According to a recent article from Law360, the letters were...
February 24, 2016
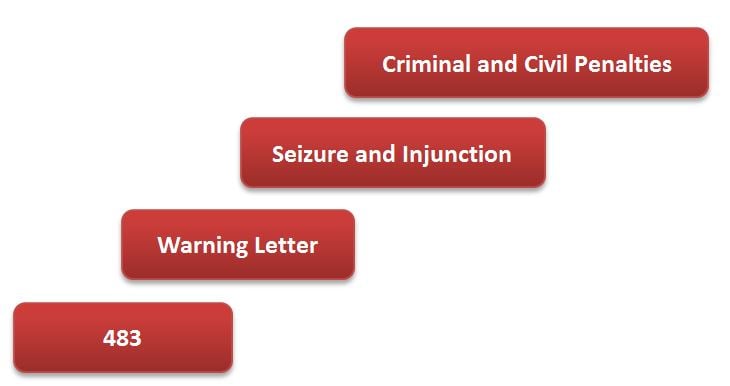
Starting with the issuance of a 483, the stepwise FDA enforcement process can be illustrated as follows: Given the seriousness of a Seizure and Injunction scenario, not to mention potential jail time...
June 21, 2016
On Wednesday, June 8th, the FDA issued a warning letter to Whole Foods Markets Inc., citing the company’s Everett, Massachusetts manufacturing facility for several GMP violations. The violations...