Everything we do today is traceable. The integrity of the data, documents and decisions needs to be defendable and consistent. Electronic Quality Management Systems (eQMS) are an effective way to manage quality processes and data. eQMS systems are typically implemented when most companies hit a tipping point of the magnitude of data or when regulatory authorities identify quality system gaps. There are many different eQMS systems on the market today. Each one was developed for specific applications and they all have strengths and weaknesses. This blog discusses four steps of eQMS implementation;
- URS Development
- Solution Provider Selection
- Solution Implementation
- Lifecycle Management
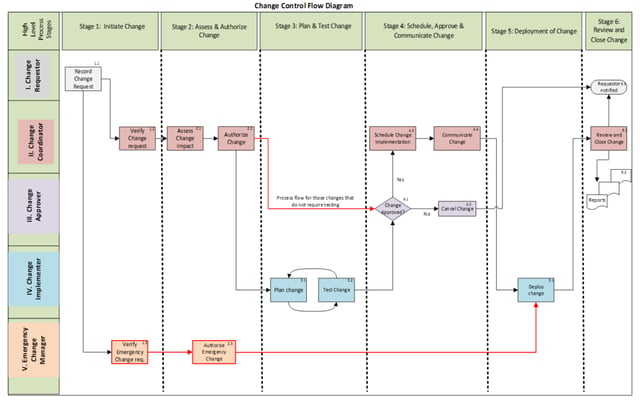
The optimal development of a URS for eQMS draws subject matter knowledge from across an organization. The key goal is to define what quality process and related data (change control, document control, risk management, training) should be required from the eQMS, how the information will be managed, who should have access, and what people with access can do. This process typically starts with mapping of the business process model for each of the workflows. Often times we find that the business process as-is state must change. Flow charts are a basic tool that allows the as-is and to-be states of information flow to be illustrated, (illustration 1).
Once the future state business model(s) have been agreed upon, the critical steps are then prioritized and given a requirement number in the URS, (illustration 2).
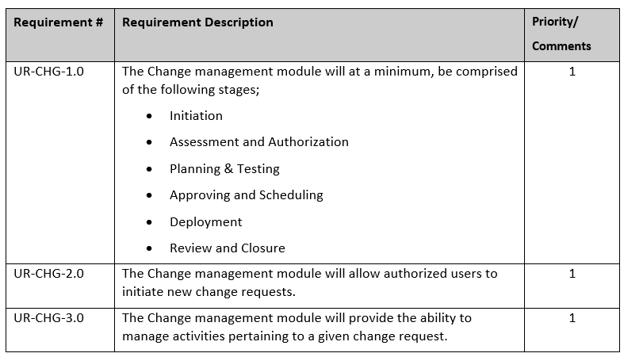 Illustration 2
Illustration 2
One of the keys of a successful URS development is to ensure a good representation of the business is included in collecting requirements that are clear, concise and traceable.
A Request For Proposal (RFP) is finalized after the URS is approved for eQMS system selection, (illustration 3).
 Illustration 3
Illustration 3
There are many eQMS solutions available. The offerings today are much more diverse than even five years ago. New development technologies and cloud based architectures introduce new opportunities as well as a few new challenges. Creation of an effective RFP will provide a standard format to assess potential supplier offerings, provide a timeframe for assessment and define the role of the solution provider. A weighted rating system (i.e. Cost may be weighted more than configuration level if funding is tight) can help your firm make sure to purchase the system that is best for you. ProPharma Group’s knowledge of the various solutions that are available brings added value to the RFP process.
Now that the Solution Provider has been selected, the eQMS implementation begins. Implementation of an eQMS system is only as good as the foundation that was established;
- Clarity given in the URS
- Effort invested into the business process models
- Resourcing appropriate individuals to the project
- Defining the roles and responsibilities of the Solution Provider
ProPharma Group has seen where Clients have purchased robust eQMS systems but underachieved the system benefits and / or missed on delivering some of the project objectives because of one or several of the points listed.
Lastly, eQMS systems have a lifecycle and the team needs to plan ahead for the different phases;
- Go-Live: Has all user acceptance testing, performance testing been completed?
- Hypercare: What are the milestones that need to be met before the solution provider / business partners are to be rolled-off the project?
- Sustaining: How often will upgrades be scheduled? Does the eQMS vendor provide a validation package for each upgrade?
- Retirement: Is there a data archival process already in place for eventual retirement of the system and data retention?
In closing, the process of identifying business processes, user requirements, detailing the steps for requests for proposals, final contract requirements and then the multiple workstreams for implementing can be formidable. ProPharma Group is more than capable of partnering with you to facilitate each step to a successful outcome.
TAGS: Computer Systems Validation (CSV) Life Science Consulting