November 23, 2021
November 23, 2021

Every company knows, timing is critical to success. But even the most well-designed products can be doomed with poor timing. Have you been tasked with leading the CMO selection process for your company? We’ve been in your shoes and understand just how critical this step is for future growth and success. Choosing the wrong contract manufacturer can be a disastrous move for a drug or device company, leading to delays in supplies or even jeopardizing regulatory approval.
A good selection process, with a realistic set of selection criteria is essential when choosing the right contract manufacturing partner. To help you with this process, ProPharma has developed the CMO Compass® tool. Developed by industry experts, this tool takes a systematic approach to the selection process.
No two CMOs are the same – they all have different strengths and weaknesses. Based on a series of questions, the CMO Compass serves as a guide to help match companies with the right CMO. It's quick and easy to use and can potentially save you precious time that you could be spending on the many other tasks you have on your plate.
In addition to resources, there are other risks involved in the CMO selection process. You can read more on this topic and how to minimize risk and overcoming early-phase Challenges.
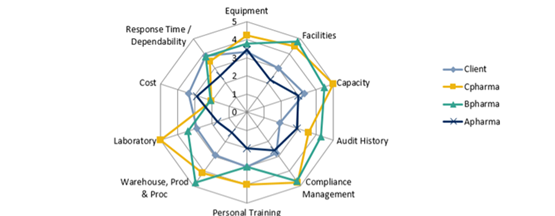
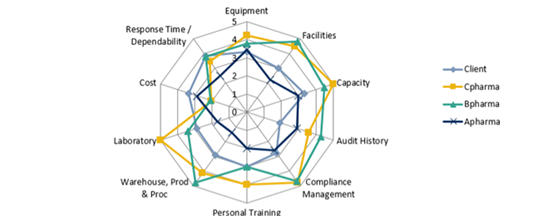
The radar graph presented below shows the different qualities that are taken into consideration during our selection process. For example, a CMO/CDMO with a strong compliance management or good audit history can help with avoiding submission rejections in later stages.
It is critical for companies to know and understand the steps required in the selection process. With that said, we advise clients to begin with the Target Product Profile (TPP). Learn more about the selection step with our blog post 5 Steps to CMO Identification and Selection for Cell and Gene Therapies.
Based on the results generated by the CMO Compass tool, clients are provided with their top CMO/CDMOs. Next, the CMO/CDMOs will be screened based on selection criteria. The initial selection criteria serves as a guide, but can be adjusted throughout the selection process if needed.
For instance, equipment and lab facilities may be the initial criteria; however, later in the process, additional criteria such as auditing history and training may be become more important and added to the selection criteria.
The next step is to “narrow the field.” Select 3-5 potential vendors to be included in the RFP process. Once the list of the CMO/CDMOs are narrowed down, ProPharma will document the decision and perform audits on your behalf. Although audits are a part of our complete quality system services, we can also work with you on an audit-only basis. The audit can be tailor-made based on your specific needs, and together we will plan your audit project plan. We will train the auditor specifically to your needs. No matter the location or type of audit, we charge a fixed price per On-Site-Audit-Day (OSAD), allowing you to control the budget.
When the audit is completed, you will receive an audit report with all findings triaged as minor, major, or critical. Once the concerns are completely recognized and understood by all the shareholders, we can start tackling them.
The next step is to have a thorough plan for technology transfer and/or scale up. Technology transfers are a crucial step in the product development process and excellence in pharmaceutical technical transfers is a “must have” for organizations today.
ProPharma uses the Nine Gate Transfer Process to ensure that each stage is completed according to agreed constraints, and allows leadership to quickly assess progress utilizing an industry approved best practice. Transfer teams are guided by trained transfer professionals with an understanding of how to maintain quality while triple constraints (Scope, Schedule, Cost) are fixed.
With more than 400 successfully performed technology transfers, our experts have created a guide to set your project up for success. Continue reading “12 Critical Questions and Answers for a Successful Tech Transfer” to learn more, or contact our experts to discover how ProPharma can support your next project.
April 5, 2021
After assuring clinical validity, finding and managing the right contract manufacturing organizations (CMOs)/contract development manufacturing organizations (CDMOs) is a Sponsor's major concern when...

June 30, 2014
Today, more pharmaceutical companies are turning to outsourcing their products as a strategic part of their business model. There are a number of drivers for this shift to soliciting the services of...

April 4, 2025
The US Government recently modified their policy to utilize tariffs to in an effort to support the US economy. Significant changes have been announced causing many global organizations to investigate...