June 28, 2022
June 28, 2022

Advanced therapy medicinal products (ATMPs) have emerged as ground-breaking therapies for rare diseases and other conditions with unmet clinical needs. As of 2022, sixteen ATMPs have been approved by the European Medicines Agency (EMA), with eleven of these having a valid marketing authorization to date. The number of ATMPs entering the market is expected to keep growing in the upcoming years.
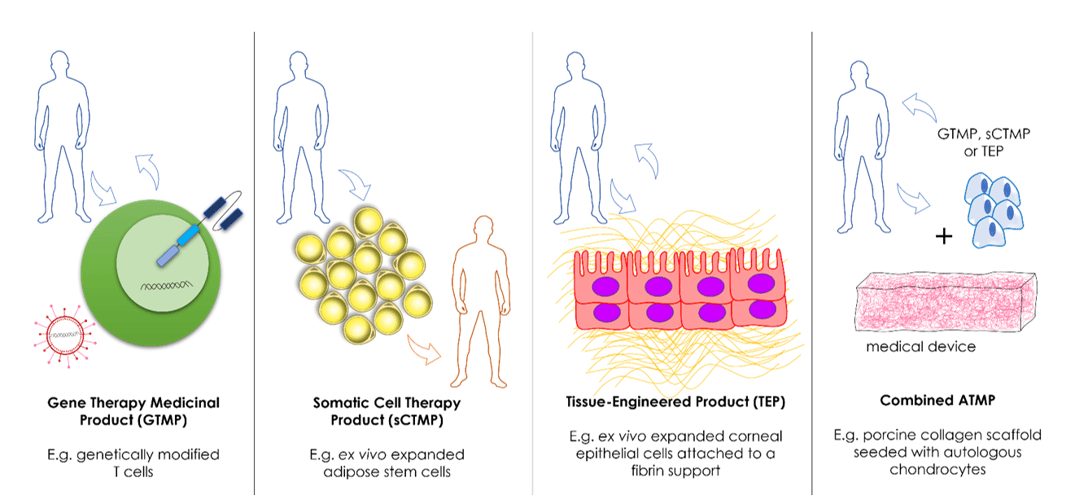
ATMPs fall under the regulatory framework of biological products (Directive 2001/83/EC), which defines the legal basis for their development. The EMA classifies ATMPs into four main types (Figure 1). Gene therapy medicinal products (GTMP) and somatic cell therapy medicinal products (sCTMP) were the first categories to be introduced, as defined in Annex I, part IV, section 2 of Directive 2001/83/EC. Two additional categories, tissue-engineered products (TEP) and combined ATMPs were later introduced in Regulation (EC) No 1394/2007. EMA’s classification is different from the US, where only two product types are recognised: gene therapy and cellular therapy products.
The classification of ATMPs in the EU has defined inclusion criteria set out in Article 2 of Regulation (EC) No 1394/2007. Here, we summarise the elements that define each ATMP category and provide some examples of products that have received full authorization in Europe.
| Category: Gene therapy medicinal product (GTMP) | |
| Description | Examples of authorised products |
| The product is a biological medicinal product containing or consisting of a recombinant nucleic acid of biological origin. The recombinant nucleic acid should be directly involved in the mechanism of action. The intended use of a GTMP is to regulate, repair, replace, add, or delete a genetic sequence. |
Strimvelis: ex vivo therapy consisting of CD34+ cells transduced with a retroviral vector for the treatment of adenosine deaminase- severe combined immunodeficiency (ADA-SCID). Kymriah: ex vivo therapy consisting of T cells transduced with a lentiviral vector for the treatment of acute lymphoblastic leukemia (ALL) and diffuse large B cell lymphoma (DLBCL). Luxturna: gene transfer vector employing an adeno-associated viral vector serotype 2 (AAV2) capsid for the treatment of vision loss due to inherited retinal dystrophy linked to biallelic RPE65 mutations. |
| Category: Somatic cell therapy medicinal product (sCTMP) | |
| Description | Examples of authorised products |
| The product consists of cells or tissues that have been subject to substantial manipulation or are not intended to be used for the same essential functions in the recipient and the donor. The intended use of a sCTMP is to treat, prevent or diagnose a disease through pharmacological, immunological, or metabolic action of its cells or tissues. |
Alofisel: expanded adipose stem cells to treat perianal fistulas in Crohn’s disease patients.
|
| Category: Tissue-engineered product (TEP) | |
| Description | Examples of authorised products |
| The product consists of engineered cells or tissues that have been subjected to substantial manipulation and that are not intended to be used for the same essential function in the recipient as in the donor. The intended use of a TEP is to regenerate, repair, or replace human tissue. |
Holoclar: ex vivo expanded autologous human corneal epithelial cells containing stem cells to treat several limbal stem cell deficiencies. Spherox: spherical aggregates of chondrocytes to repair defects in knee cartilage. |
| Category: Combined ATMP | |
| Description | Examples of authorised products |
| The product must incorporate, as an integral part, one or more medical devices and a cellular or tissue component, which contains viable or non-viable cells. | MACI: matrix-induced autologous chondrocytes to treat full-thickness cartilage defects (withdrawn). |
While it may seem to be a straightforward exercise, ATMP classification can be challenging. For instance, a product that may fall within the definition of one category can also be considered as belonging to a different one. Therefore, it is important to define the attributes of the product and provide clear scientific information on the extent of manipulation, the intended function, and the claimed mode of action (MoA).
Cells or tissues are considered “engineered” if they undergo substantial manipulation. This means that the cells/tissues have been processed in ways that alter their biological, physiological, or structural properties. Substantial manipulation includes procedures such as cell expansion, genetic modification, and cell differentiation. In the absence of substantial manipulation, a product can still be classified as ATMP if it is intended to perform a different function (non-homologous) in the donor and the recipient. For example, traditional bone marrow transplants are not ATMPs because the cells/tissues are minimally manipulated and used for the same essential function when grafted; however, if the bone marrow cells are used for cardiac repair in the recipient, they can, under these circumstances, be considered ATMPs.
The claimed MoA is closely linked to the product’s intended function and is crucial for defining ATMPs. For instance, a product will be considered GTMP only if the nucleic acid sequence or its product is directly involved in the MoA. Importantly, a clear distinction between prophylactic and therapeutic functions has to be established for potential GTMPs, as products with prophylactic MoA, such as vaccines, must not be included in this category. Furthermore, the MoA is the main differentiator between sCTMPs and TEPs since these two categories share the same inclusion principles. Some general premises and workflows have been drafted to facilitate the categorisation exercise, but the final ATMP classification should be considered on a case-by-case basis.
The ATMP classification procedure is free of charge and legally non-binding. To apply, two documents must be completed: an administrative form and a classification request and briefing information document. The latter will contain information on the product and its development, including manufacturing and quality aspects, as well as an outline of the non-clinical and clinical development work. Applications must be submitted following the allocated deadlines published on the EMA’s website.
ATMP classification requests are received by the EMA at the latest 15 days before the start of the procedure. This allows for the inspection of the submission package, and requests for additional information if needed. This is followed by a draft preparation period by the Committee for Advanced Therapies (CAT), which is the body responsible for the scientific recommendation on ATMP classification. The CAT then holds a meeting to discuss the draft scientific recommendation and delivers the outcome after consultation with the European Commission (EC), all this within 60 calendar days after receipt of the request.
Companies can consult EMA to determine whether a therapy meets the scientific criteria for defining an ATMP. This procedure is optional, but it represents a unique opportunity to address questions on borderline products and to initiate early interactions with regulators. This can then lead to the use of other regulatory procedures, such as scientific advice and ATMP certification (only for small and medium enterprises, SMEs). The ATMP classification may also help developers gain access to relevant services and incentives offered by the EMA.
Developers can apply at any point during product development, but it is recommended that ATMP classification is done before submission of requests for scientific advice/protocol assistance, Paediatric Investigation Plan (PIP) evaluation, certification of quality and non-clinical data for SMEs developing ATMPs, orphan drug designation (ODD), and Marketing Authorisation Application (MAA).
At ProPharma Group we know that the correct classification of a product at an early stage of development is critical, as this will determine the regulatory framework and recommendations to follow throughout the product lifecycle. Our consultants have years of experience and have learned that guiding clients through the ATMP classification process can have great benefits, including legal and scientific feedback and access to expedited development programs.
Interested in learning more? Contact us today to find out how we can help not only with your ATMP classification submission to the EMA, but to learn more about all our global regulatory services.
TAGS: Europe Regulatory Sciences
August 22, 2022
Orphan Designation of ATMPs for Rare Diseases: MPS II Case Study Many advanced therapy medicinal products (ATMPs) in development in the EU are for rare diseases and conditions. Since the...
August 26, 2020
As the field of modern medicine is changing, so should the development strategies of these new therapies such as cell and gene therapy (CAGT) products, also known as advanced therapy medicinal...