May 30, 2019
May 30, 2019
At the beginning of each federal fiscal year, the US FDA posts the previous year's Form 483 observation metrics issued by each product center.
Reviewing these metrics provides a valuable snapshot of industry's compliance direction. Understanding these common issues can help highlight focal areas when evaluating potential quality management system (QMS) gaps and improvement opportunities within your company.
Summaries of the top five observation occurrences reported in Form 483s per product center are as follows:
The 2018 top 5 observations reported by the Drug sector are:
The 2018 top 5 observations reported by the Devices sector are:
The 2018 top 5 observations reported by the Biologics sector are:
The 2018 top 5 observations reported by the Veterinary sector are:
Focusing on the Drug Sector, the top five observations reported by FDA over the last three years are as follows:
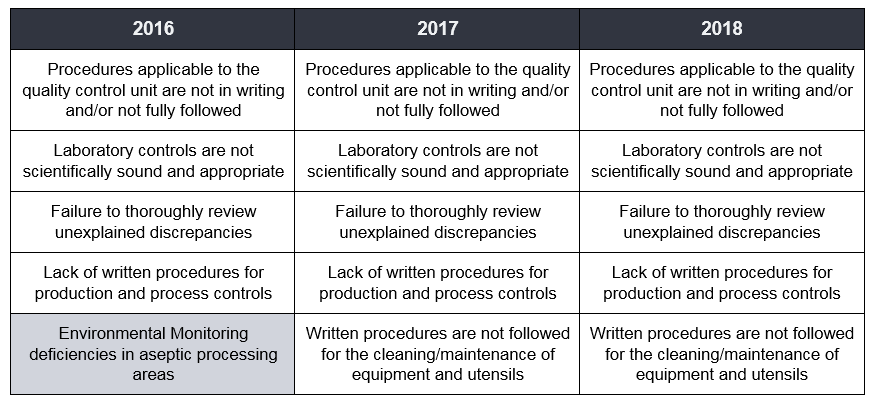
This data shows that the FDA is encountering the same issues year-over-year with the exception of the fifth place observation in 2016. As an industry, we should be prepared for FDA's increased focus on these recurring themes.
I find that a good starting point for risk reduction is understanding industry's trouble areas. One way to approach this is by taking a closer look at the governing quality systems that cover the top ranking observations. For instance, review the thoroughness of your site's training program and simplify complex or confusing SOPs to help avoid a "procedures not followed" observation. To prevent observations relating to "inadequate investigations", look to Root Cause Analysis procedures for possible gaps. Monitoring the status of your site's QMS and reacting to reported quality metrics helps to avoid these common observations and facilitates continuous improvements. Has a holistic gap assessment recently been performed on your firm's QMS? Are your operations susceptible to these common findings?
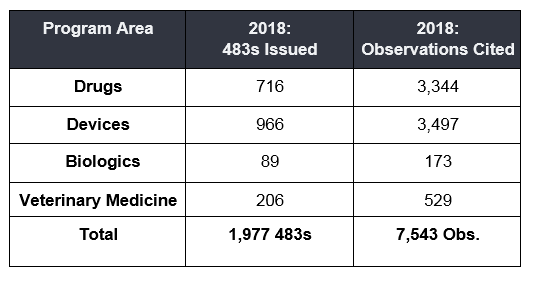
Evaluating another aspect of the data, nearly 2,000 Form 483s were issued by FDA's Office of Regulatory Affairs in 2018 across the following four FDA regulated industries, Drug, Device, Biologics, and Veterinary. As seen in the graphs below, the total number of Form 483's issued by inspectors hasn't changed drastically across the sectors in the past five years, neither has the average number of observations cited per each 483 issued.
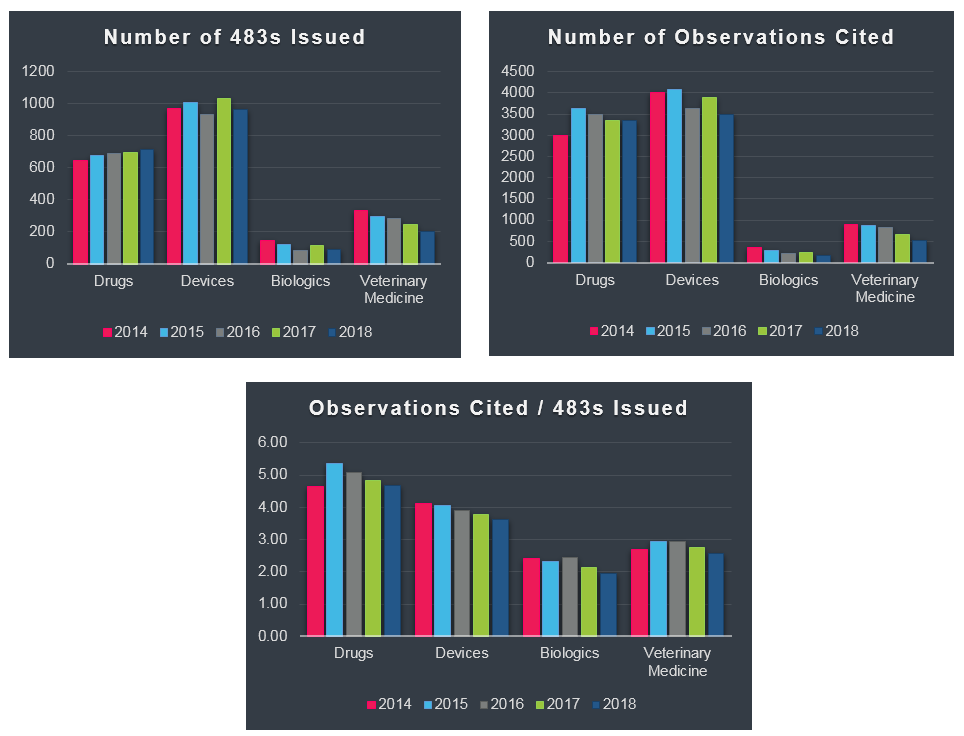
What do these observation numbers suggest, what can we learn from this data, is your site at risk for one of these observations? As an industry what can be done to help avoid receiving one of these common observations? Is industry compliance not improving?
To fully answer these questions and measure industry's overall compliance health, one needs to know the total number of inspections performed for each sector. Are there now fewer inspections and a higher rate of 483s issued or more inspections and a lower rate of 483s issued? Is industry improving quality but inspectors applying more scrutiny?
While the regulations haven't changed drastically in recent years, the compliance expectation is ever increasing. FDA utilizes computerized applications that allow inspections to be more risk-based and better focus inspections on industry trends, guiding inspectors towards common noncompliance areas within industry quality systems. The trend shows not following procedures, inadequate record handling, and inadequate investigations and CAPA assignment are frequent themes in industry.
I like to keep common industry pitfalls in mind when evaluating the capability and key quality indicators of a quality system, this helps me determine whether a site has a good handle on their quality system and that its state of control is maintained throughout product lifecycle. This allows me to gauge how effectively the facility manages risks or how capably the facility can react to unforeseen issues. This approach helps me direct the quality system towards improvement and increased compliance.

April 21, 2023
This article has been updated since its original publication date. FDA Form 483 requires a written response in which you must make it clear that you are taking the observations, and your...
November 13, 2013
In the just issued Johnson & Johnson Corporate Integrity Agreement (CIA), the Office of Inspector General (OIG) has, for the second time in less than a year, required that a company maintain a...
January 10, 2018
At the beginning of each federal fiscal year, the US FDA posts the previous year’s Form 483 observation metrics issued by each product center. I find that reviewing these metrics provides a valuable...