At the beginning of each year, the US FDA posts the previous year’s Form 483 observation metrics issued by each product center.
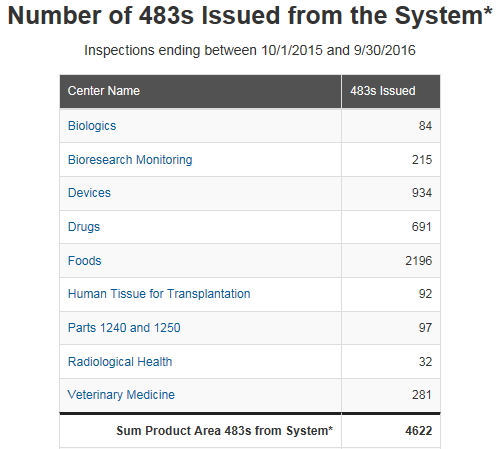
Image Source: FDA
I find that reviewing these metrics provides a valuable snapshot of industry’s compliance direction. Understanding these common issues can help highlight focal areas when evaluating potential quality system gaps and improvement opportunities within your company.
Summaries of the top five observation occurrences per sector are as follows:
The 2016 top five observations reported by the Drug sector are:
- Procedures applicable to the quality control unit are not in writing and/or not fully followed
- Laboratory controls are not scientifically sound and appropriate
- Failure to thoroughly review unexplained discrepancies
- Lack of written procedures for production and process controls
- Environmental Monitoring deficiencies in aseptic processing areas
The 2016 top 5 observations reported by the Devices sector are:
- Procedures for corrective and preventative action have not been adequately established
- Procedures for receiving, reviewing, and evaluating complaints have not been adequately established
- Written MDR procedures have not been developed and/or established
- Procedures have not been adequately established to control product that does not conform
- Procedures to ensure that all purchased or received products/services conform to specifications have not been adequately established
The 2016 top 5 observations reported by the Biologics sector are:
- Written standard operating procedures were not always established or available
- Records are not concurrently maintained with the performance and traceability of each significant step
- Failure to perform a thorough investigation
- Records fail to provide a complete history of the work performed
- Failure to submit a product deviation report for a reportable event
The 2016 top 5 observations reported by the Veterinarysector are:
- Treatment records were not maintained
- Causing a residue of an approved human or animal drug above an established safe level
- Lack an adequate inventory system for quantities of drugs used to medicate livestock
- Failure to maintain records regarding the identity of animal(s)
- Use of drug in a manner contrary to label directions without valid veterinary direction
Nearly 2,000 observations were issued by FDA in 2016 across the four FDA regulated industries listed above, Drug, Device, Biologics, and Veterinary. What do these numbers imply, what can we learn from this data, is your site at risk for one of these observations? As an industry what, can be done to help avoid receiving one of these common observations?
While the regulations haven’t changed drastically in recent years, the compliance expectation is ever increasing. FDA utilizes computerized systems (e.g., Turbo EIR) that allow inspections to be more risk-based and better focus inspections on industry trends, guiding inspectors towards common noncompliance areas within industry quality systems. One observation is that there are common trouble spots overarching industry. The trend shows that deviations handling, investigations and CAPA are recurring through pharma, device, and biologics.
As an industry, we should be prepared for FDA’s increased focus on these reoccurring themes. I find that a good starting point for risk reduction is understanding industry’s trouble areas. One way to approach this is by taking a closer look at the governing quality systems that cover the top ranking observations. For instance, review the thoroughness of your site’s training program and/or simplify complex or confusing SOPs to help avoid a “procedures not fully followed” observation. Preventing observations relating to “inadequate investigations” look to adverse event, deviation management, and CAPA procedures for possible gaps. Monitoring the status of your site’s quality systems and reacting to reported quality metrics helps to avoid these common observations and facilitates continuous improvements.
I like to keep common industry pitfalls in mind when evaluating the capability and key quality indicators of a quality system, this helps me determine whether a state of control is maintained throughout product lifecycle, and gauge how effectively the facility manages risks. This practice helps me direct the quality system towards improvement and increased compliance.
When reflecting on last year’s top issues, thoughts of the famous quote by George Santayana are evoked, “Those who cannot remember the past are condemned to repeat it.”
Learn more about ProPharma's Compliance services. Contact us to get in touch with our subject matter experts for a customized presentation.
TAGS: Compliance Qualification & Validation FDA Form 483 Life Science Consulting
