June 2, 2022

June 2, 2022

FDA has announced the availability of a proposed rule National Standards for the Licensure of Wholesale Drug Distributors (WDDs) and Third-Party Logistics Providers (3PLs).
The proposed rule sets standards for federal and state licensure for WDDs and 3PLs.
The draft’s intent is to provide greater assurance that supply chain participants are qualified to distribute prescription drugs to strengthen the supply chain.
Currently the requirements for wholesale drug distributors vary between states. Those requirements and standards for licensure vary on different topics, such as. All this leads to different regulations.
The current version of 21CFR205 is not very extensive and is divided as:
The proposal is much more comprehensive and contains a series of very detailed requirements as:
Subpart A—Third-Party Logistics Providers Licensure Standards
Subpart B—Approved Organizations for 3PLS
Subpart C—Wholesale Distributors Licensure Standards
Subpart D—Approved Organizations for Wholesale Distributors
205.32 – General qualifications of approved organizations
205.33 – Process and procedures for approval by the Food and Drug Administration
Thus, the new Part 205 would implement licensure requirements for Drug Supply Chain Security to further improve security of track trace by providing oversight of licensure of 3PLs and WDDs. Where, 3PL facilities would be required to obtain a 3PL license for each facility. And each WDD would be licensed by the State or the FDA.
Follow the below link for more details https://www.federalregister.gov/documents/2022/02/04/2022-01929/national-standards-for-the-licensure-of-wholesale-drug-distributors-and-third-party-logistics#sectno-reference-205.1
TAGS: Life Science Consulting
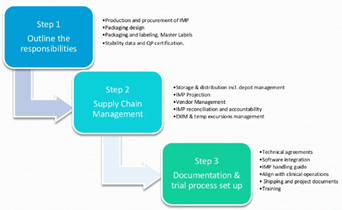
October 20, 2022
Things to consider and how to ease the process IMP Supply Management is a journey where GMP meets GCP and GDP. This journey includes finance, flow of products and documentation. How a company manages...

August 30, 2022
Updates have been announced by FDA and for USP <1079>. In this blog we cover these changes. USP USP <1079> has a series of chapters on Good Storage and Distribution Practices. Chapter <1079> applies...
October 10, 2012
One of the challenges to starting any User Requirement Specification (URS) is to envision a structure which can allow for traceability as the project continues. The attached sample URS is a starting...