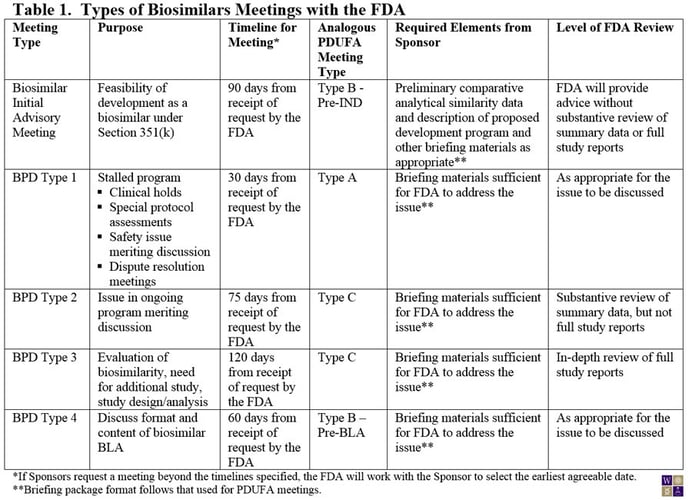
On March 29, 2013, the FDA made available a draft guidance for Sponsors of biosimilar products outlining the procedures and processes for meetings with the FDA. Although much of guidance for the biosimilar biological product development (BPD) program closely aligns with the current guidance for PDUFA meetings, experience from meetings with biosimilar product Sponsors led to the conclusion that a somewhat different framework was needed for biosimilar products (Table 1). For example, the need for preliminary comparative analytical data for an initial meeting with the FDA is now specified. Also, the PDUFA type C meeting has been broken into two subtypes: Those that require an in-depth review of study reports from the sponsor (BPD Type 3) and those that can be addressed through review of summary data (BPD Type 2). Where in-depth review of study reports is needed, the FDA has allowed extra time for their internal review.
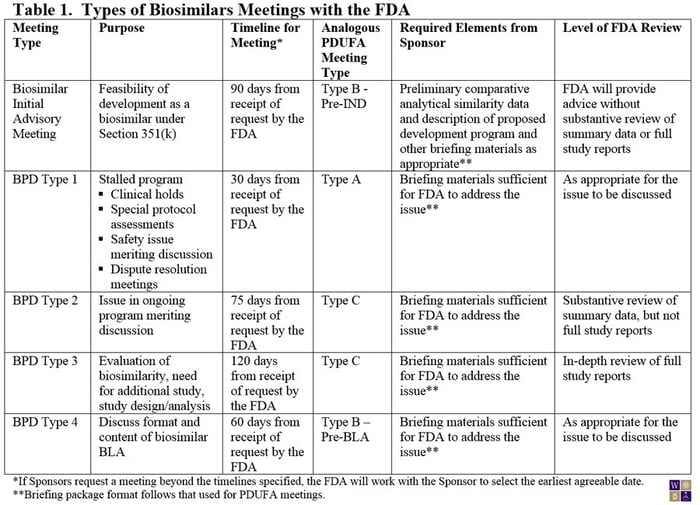
Notably, unlike the PDUFA meetings guidance, the FDA requires that the briefing package for the meeting be submitted at the time of the meeting request or the proposed meeting will be denied.
The fee schedule to pay for participation in the BPD program is also described in the draft guidance. Although there is no fee to participate in a Biosimilar Initial Advisory Meeting with the Agency, further interaction triggers the fees which are assessed on a per-product basis:
Initial fee: Due on the date the IND for the biosimilar is filed or within 5 days of the FDA granting a BPD Type 1, 2, 3, or 4 meeting (whichever occurs first).
Annual fee: Due each year until a BLA is filed for the biosimilar or the Sponsor discontinues participation in the program.
Reactivation fee:For Sponsors who have discontinued participation; due on the date the IND is filed or within 5 days of the FDA granting a BPD Type 1, 2, 3, or 4 meeting (whichever occurs first).
The FDA reserves the right to cancel any meeting for which the Sponsor fails to pay the applicable fee in the time specified or to refuse to grant a meeting for a Sponsor who is in arrears for their fees. The guidance is silent on whether an IND will be accepted if the appropriate fees have not been paid, but FDASIA Section 744H(a)(1)(E) indicates that an IND will be considered incomplete and not accepted for review if the fee is not paid.
If you have any questions or thoughts on this blog post or others, please contact us.