The accelerated approval provisions of FDASIA in section 506(c) of the FD&C Act provide that FDA may grant accelerated approval to:
. . . a product for a serious or life-threatening disease or condition . . . upon a determination that the product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments (FDA, 2014).
Accelerated approval can provide important benefits to a Sponsor. Most importantly, a drug or biologic can be approved earlier than through a traditional approval pathway. However, continued approval by the FDA requires verification of clinical benefit through a rigorous post marketing study.
FDA Accelerated Approval Analysis
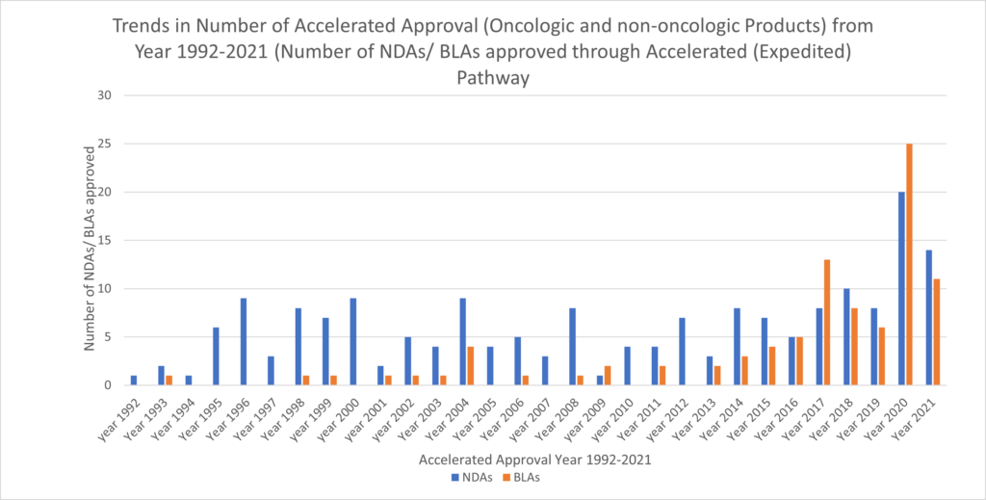
Information on the FDA website titled “CDER Drug and Biologic Accelerated Approvals Based on a Surrogate Endpoint” (FDA, 2022a) was utilized to find the number of New Drug Applications (NDAs) and Biologics License Applications (BLAs) approved via Accelerated Approval (AA) from the year 1992 to 2021. Figure 1 shows the trends of NDAs and BLAs (oncologic and non-oncologic products) approved through AA. For both drugs and biologics, there has been significant variability over time in the number of approvals. The greatest number of accelerated approvals for both drugs and biologics occurred in 2020 at 20 and 25 respectively. In 2021, the number of accelerated approvals decreased for both drugs and biologics with biologics having the larger decrease.
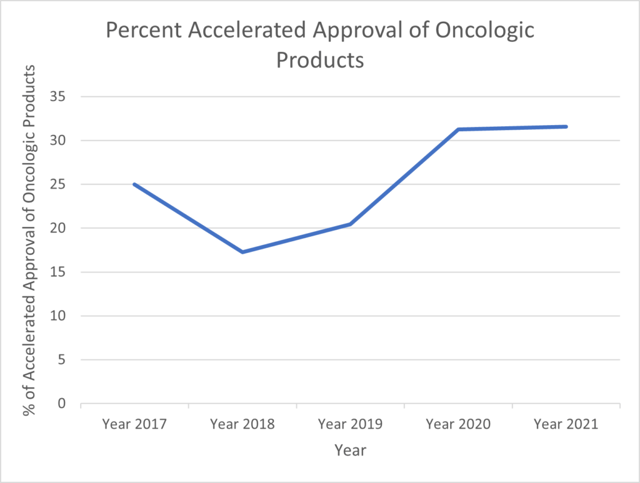
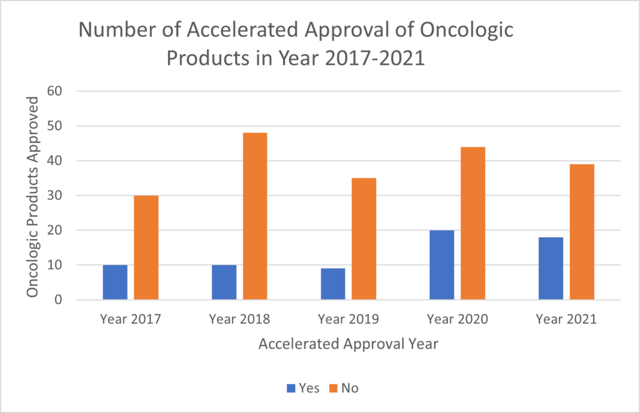
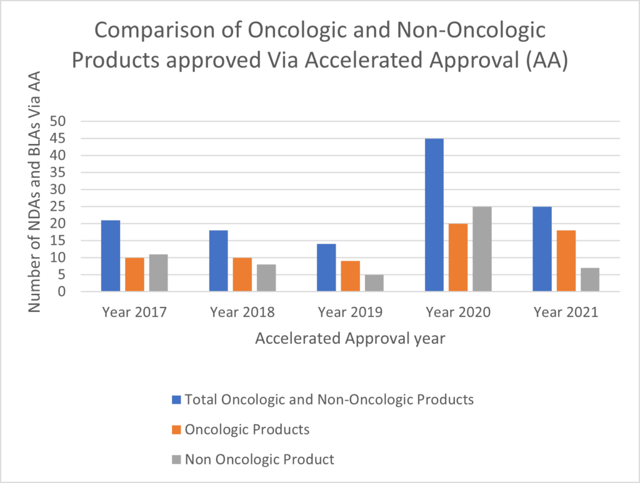
For this analysis, the ProPharma group focused on oncology approvals. The FDA website for Oncology (Cancer)/ Hematologic Malignancies Approval Notifications (FDA, 2022b) was exported and utilized to find the number of oncological products that have been approved via AA from the year 2017 to 2021. The number of oncological products approved via AA and Regular approval were counted and plotted. Among the data set, approvals for, diagnostic devices, biosimilar products and new dosage forms were excluded from computation. Figure 2 shows the percent of accelerated approvals of oncologic products through Year 2017 to Year 2021. The percentage decreased from 2017 to 2018 followed by an increase until 2020. In 2021, the percentage stands at 32%. Figure 3 shows the comparison of number of accelerated approvals and regular approvals for oncologic products for the year 2017-2021. Figure 4 shows comparison of oncologic products, non-oncologic products and Total products approved via AA.
Conclusion
The overall number of AAs decreased substantially in year 2021 as compared to 2020. Annual AAs in oncology were substantially greater than that for non-oncology indications in 2021. In 2021, AAs in oncology accounted for 32% of overall oncology approvals. ProPharma has substantial expertise in helping clients decide whether AA is a reasonable pathway to consider as well as in submitting a NDA or BLA for AA.
Interested in learning more? Contact us today to find out how we can help with your global regulatory needs.
References
FDA (2014). Guidance for Industry Expedited Programs for Serious Conditions – Drugs and Biologics.
FDA (2022a). CDER Drug and Biologic Accelerated Approvals Based on a Surrogate Endpoint.
FDA (2022b). Oncology (Cancer)/ Hematologic Malignancies Approval Notifications.