September 19, 2024

September 19, 2024

Welcome to the Diary of Compliance, where we follow the journey of Allison Audit, the dedicated and detail-oriented Quality Assurance Manager at Acme Pharma. Over the course of a year, Allison takes us through the highs and lows of ensuring her company meets the rigorous FDA CAPA (Corrective and Preventive Actions) requirements. From initial resolutions to unexpected challenges and triumphant inspections, join Allison as she navigates the intricate world of pharmaceutical compliance and provides quarterly reports on the company progress.
As the calendar turns to a new year, Acme Pharma has set a bold resolution: to overhaul our FDA CAPA system and ensure we meet and exceed compliance standards. Leading this charge is yours truly, Allison Audit, with a dedicated team ready to embrace the challenge.
We kicked off the year with a comprehensive team meeting to establish our goals. Our CEO, Mr. Pillster, emphasized the importance of quality and patient safety, setting the tone for our mission. We decided that our first major task would be conducting an internal audit to identify gaps in our current FDA CAPA processes.
The internal audit was a revealing exercise. We discovered several areas needing improvement, from incomplete documentation to neglected corrective actions. This audit highlighted the necessity of a robust FDA CAPA system and set the stage for our action plan.
With the audit findings in hand, our next step is to develop and implement a comprehensive corrective action plan. The plan will address the identified gaps and strengthen our FDA CAPA processes. As we move forward, we’re committed to making Acme Pharma’s FDA CAPA system more effective and ensuring continuous compliance with FDA standards.
As the new year unfolded, the first quarter at Acme Pharma was dedicated to diving deep into the FDA CAPA requirements. With our ambitious resolution to overhaul our FDA CAPA system, the initial focus was on building a solid foundation of knowledge and understanding.
Our team spent the first few weeks immersed in regulatory documents and guidelines, dissecting each aspect of the FDA CAPA requirements. Key documents included:
We held several workshops to break down the key components of an FDA CAPA system, emphasizing the importance of thorough root cause analysis, effective corrective actions to avoid recurrence of issues, and preventive actions to eliminate the causes of potential nonconformities to prevent their occurrence. We discussed the necessity of having documented procedures for identifying quality problems, investigating their causes, and implementing corrective and preventive actions to address these issues and prevent them from recurring.
During these sessions, I could see the team’s initial apprehension transform into a determined resolve. We knew that mastering these requirements was crucial to developing a robust corrective action plan that would not only meet but exceed FDA standards.
With a firm grasp of the FDA CAPA regulations, we set out to develop our corrective action plan. This was no small task, as it required us to meticulously map out each step, assign responsibilities, and set realistic deadlines.
We started by addressing the findings from our January internal audit. The gaps identified provided a clear roadmap for our corrective action plan. We held brainstorming sessions to devise practical solutions for each issue, ensuring that our plan was comprehensive and actionable.
One of the critical elements of our plan was assigning clear ownership for each corrective action. This not only ensured accountability but also empowered team members to take charge of specific areas, fostering a sense of ownership and responsibility.
We also introduced regular training sessions to ensure everyone was on the same page. These sessions covered the fundamentals of FDA CAPA standards and specific elements of our corrective action plan. The team’s enthusiasm during these sessions was contagious, and it was clear that we were all committed to making this plan a success.
By the end of the quarter, we moved from planning to action. The implementation of our corrective action plan began in earnest, with the team rolling up their sleeves and diving into the work.
We updated our documentation practices to ensure thorough and accurate records, which are vital for both internal tracking and FDA inspections. We also introduced CAPA software to streamline our processes, making it easier to track progress and ensure nothing fell through the cracks.
Of course, the road wasn’t entirely smooth. We encountered some initial resistance to the changes, which is natural in any organization. To address this, we held additional training sessions and open forums for feedback, allowing team members to voice their concerns and suggestions.
Technical issues with the new software were another challenge. We worked closely with the vendor to resolve these issues quickly, ensuring that the tools we had in place were effective and user-friendly.
Despite these hurdles, the early results of our implementation were promising. We saw immediate improvements in our documentation and the efficiency of our corrective actions. The team’s dedication and adaptability played a significant role in these early successes, and I couldn’t be prouder of their efforts.
The second quarter marked a critical phase in our journey at Acme Pharma as we moved from initial implementation to full-scale execution of our corrective action plan. With the foundational work completed in Q1, we were ready to roll out the plan across all departments and begin monitoring our progress closely.
Our first task was to ensure that every team member fully understood their role in the corrective action plan. We conducted department-specific training sessions to reinforce the importance of adhering to FDA CAPA requirements and how each individual's actions contributed to the overall success of the plan. This targeted approach helped to address specific concerns and challenges faced by different departments, ensuring a smooth rollout.
One of the key components of our corrective action plan was the introduction of regular CAPA reports. These reports provided a structured way to document and track corrective and preventive actions, ensuring transparency and accountability at every step. Each report included detailed information on identified issues, root cause analyses, corrective actions taken, and preventive measures implemented.
We standardized the format of our CAPA reports to align with FDA expectations, ensuring that all necessary details were captured. This standardization not only made it easier for us to track and review progress but also prepared us for potential FDA inspections.
With the corrective action plan in full swing, monitoring and reviewing progress became our primary focus. We established a CAPA review board consisting of representatives from each department. The board meets on a routine basis to review CAPA reports, discuss ongoing issues, and assess the effectiveness of implemented actions.
During these meetings, we identified patterns and recurring issues that required additional attention. For example, we noticed that certain types of nonconformities were more frequent in specific departments, indicating a need for targeted preventive measures. By addressing these trends proactively, we were able to prevent small issues from escalating into major problems.
Despite the challenges, we achieved several early successes in Q2. Our updated documentation practices and CAPA reports led to more accurate and timely recording of corrective actions. We also saw improvements in product quality and a reduction in recurring issues, demonstrating the effectiveness of our preventive measures.
The team’s commitment to continuous improvement was evident throughout the quarter. We encouraged feedback and suggestions for further refining our processes, fostering a culture of collaboration and innovation. This proactive approach not only strengthened our CAPA system but also empowered team members to take ownership of their roles in ensuring compliance and quality.
The third quarter at Acme Pharma was dedicated to refining our processes and ensuring that our corrective action plan was as robust as possible. Building on the progress made in Q2, we focused on addressing any remaining gaps and reinforcing our commitment to continuous improvement.
Our CAPA review board continued to play a crucial role in monitoring progress and identifying areas for improvement. During Q3, the board conducted an in-depth analysis of the CAPA reports from the previous quarters, focusing on recurring issues and areas where corrective actions were less effective.
We used the insights gained from this analysis to adjust our corrective action plan. For instance, we identified a need for more comprehensive training in specific departments where nonconformities were still prevalent. By tailoring our training sessions to address these specific needs, we aimed to enhance the overall effectiveness of our preventive measures.
One of the key lessons learned during Q3 was the importance of robust preventive actions. While our corrective actions were effective in addressing existing issues, we realized that stronger preventive measures were needed to avoid future problems.
To this end, we revisited our root cause analysis processes, ensuring that they were thorough and that all potential causes were identified and addressed. We also implemented more rigorous risk assessments, aligning with ICH Q9 guidelines for Quality Risk Management. This proactive approach helped us to identify and mitigate risks before they could impact product quality or compliance.
Q3 also brought the challenge of preparing for regulatory audits and inspections. We knew that demonstrating our commitment to FDA CAPA requirements and our continuous improvement efforts would be critical during these audits.
Our preparations included comprehensive internal audits, focusing on the areas most likely to be scrutinized by regulators. We conducted mock inspections to ensure that our documentation, processes, and team members were all prepared to meet FDA expectations. These efforts paid off when we successfully navigated a surprise FDA inspection with minimal findings, a testament to the strength of our CAPA system.
Technology continued to play a vital role in our CAPA processes. During Q3, we further optimized our CAPA software to improve data accuracy and reporting capabilities. We also explored advanced analytics tools to gain deeper insights into our quality data, helping us to identify trends and areas for improvement more quickly.
One significant enhancement was the integration of our CAPA software with other quality systems, such as document management and training platforms. This integration streamlined our processes, reduced the risk of errors, and ensured that all quality-related activities were aligned and cohesive.
As the year drew to a close, the fourth quarter at Acme Pharma was focused on reviewing our progress, celebrating our achievements, and planning for the future. Our commitment to FDA CAPA requirements had driven significant improvements throughout the year, and it was time to consolidate our gains and set the stage for continued success.
Our first major task in Q4 was conducting a comprehensive year-end audit. To ensure the highest level of objectivity and expertise, we engaged third-party consultants to assist with our internal auditing and inspection readiness work. These consultants brought fresh perspectives and specialized knowledge, helping us identify areas for improvement that we might have overlooked.
The year-end audit revealed several key findings:
The third-party consultants played a crucial role in enhancing our audit processes. They provided valuable insights and recommendations based on their extensive experience in the pharmaceutical industry. Their involvement ensured that our internal audits were rigorous and comprehensive, preparing us effectively for any regulatory inspections.
The consultants also conducted mock inspections, simulating FDA audits to test our readiness. These exercises were invaluable, allowing us to identify and address potential weak points in our processes before the actual inspections. The feedback from these mock inspections helped us fine-tune our corrective action plan and ensure all team members were well-prepared and confident.
With the audit completed, it was time to celebrate our achievements. We held a year-end town hall meeting where we shared the audit findings, highlighted key successes, and recognized the hard work and dedication of our team. This celebration was a chance to acknowledge our accomplishments and reinforce our collective commitment to continuous improvement.
Looking ahead, we know that our journey is far from over. The insights gained from our year-end audit provided a clear roadmap for future improvements. We developed an action plan for the coming year, focusing on several key areas:
Reflecting on the past year, we are proud of the progress we have made in enhancing our FDA CAPA system and overall quality management. The journey has not been without its challenges, but the dedication and resilience of our team, along with the support of our third-party consultants, was instrumental in overcoming them.
As we look to the future, our commitment to quality and compliance remains unwavering. We know that maintaining and improving our corrective action plan is an ongoing process, requiring continuous vigilance and adaptability. However, with the strong foundation we have built, we are confident in our ability to meet these challenges and achieve even greater success.
While the story of Allison Audit and Acme Pharma is a work of fiction, it reflects the genuine efforts and challenges companies face in adhering to FDA CAPA requirements. ProPharma offers tailored consulting services to help life science companies manage FDA CAPA compliance and improve their quality systems. Our experienced team is ready to assist with audits, training, and more, ensuring your organization stays on track.
Reach out to ProPharma for expert guidance and support. We're here to help you achieve compliance excellence and maintain the highest standards of quality.
TAGS: Quality & Compliance FDA CAPA

August 1, 2023
Welcome to Part 2 of our blog series on "What You Need to Know About GxP Independent Compliance Audits." In Part 1, we discussed the importance of GxP audits, the different audit types, and why...
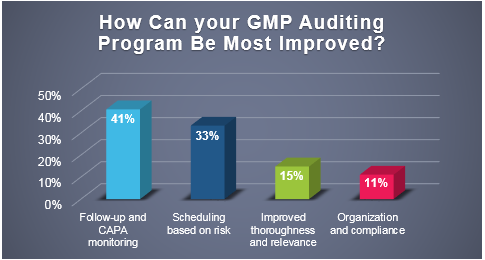
November 29, 2018
Recently, ProPharma conducted a poll to quality professionals across the country to understand the challenges that FDA regulated companies face in managing their GMP auditing programs. As depicted in...