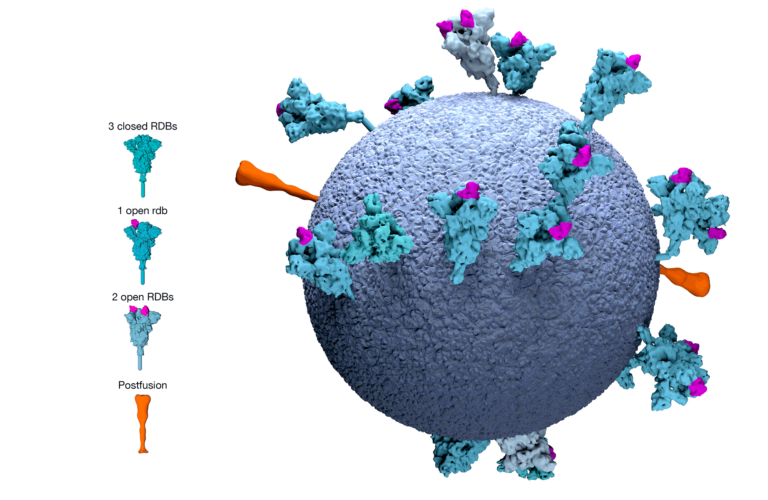
EMA adopts first list of critical medicines for COVID-19
News On 7 June 2022, EMA's Medicines Shortages Steering Group (MSSG) adopted the list of critical medicines for the COVID-19 public health emergency. The medicines included in the list are authorised...
COVID-19 Pandemic in the EU: Fraudulent Medicines, Test Kit Shortage, & EMA Guidance Regarding Ongoing Clinical Trials
As of March 27, 2020 Each health authority within the EU has information regarding COVID-19 (coronavirus) on its website. In this blog we will try to guide you through some of these recommendations...
FDA’s Breakthrough Therapy Designation vs PRIority MEdicines (PRIME) Application in Europe
What are Breakthrough Therapy Designation and PRIority MEdicines (PRIME) Applications? The advancement of modern medicine, and the accessibility of researched and regulated medication, has greatly...