Over recent years relations with data and its weight have changed significantly; consequently, an assessment of Company Data Integrity is becoming one of the key metrics of corporate functionality and effectiveness.
The state of completeness, consistency, timeliness, accuracy, and validity that makes data appropriate for a stated use is nowadays regarded as Data Integrity (DI). From this definition, it can be concluded that DI more than supports business processes. It is in fact integrated data that controls the processes and acts as input for continuous improvement. In addition, DI is employed to support compliance evidence during audits.
Data Integrity as a Structure
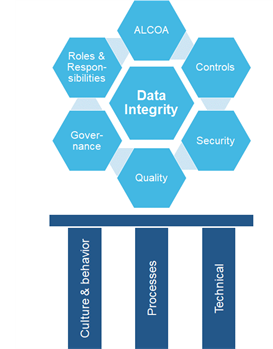
DI can be viewed as a structure that must be built with strong and durable materials, most like the Sagrada Família, not taking as long to build but a cultural foundation is not set in a day. In order to understand this, take a look at the illustration below. The bottom gives the three pillars with “Culture & Behavior” being the most important. Like introducing the Lean way of working into a culture. It takes time and openness and a safe environment to introduce, accept, and cultivate. This is a key success factor upon introducing something new that transforms company culture. Apply Moonshine teams as a tool and method to innovate and create customized solutions. These teams catalyze motivation and dedication.
Once that’s on track, the process and technical pillars can be built out of a solid cultural foundation. Existing processes are redefined and new ones introduced. And last of all on a technical level, systems are modified or introduced to support the processes.

Regulation by Agencies and Authorities
Legislation provides a framework while guidelines show the current situation and the way forward. Though this might sound easy, it is first the care and custody of all individuals handling data, and thus the culture, that is the determining factor. In addition, procedures and systems in place to control and protect data are crucial. But without the mindset and dedication of people, this is a useless effort. Consequently, human interaction is the strongest and weakest link.
Organizations measure the level of compliance to the regulatory requirements regarding data integrity.
Data Integrity Maturity Model as an Assessment Tool
What is the current state of your data integrity program? In most cases, one or more DI element(s) will already be in place. It may be a very simple set of processes and programs, or a more advanced, strategic combination. Determining the current state helps to define the maturity level and starting point. From there, improvement measures and next steps are identified.
The maturity model classifies the company’s activities in 6 different areas relevant to data integrity:
- Culture
- Governance and organization
- Strategic planning and data integrity program
- Regulatory
- Data life cycle
- Data life cycle - supporting processes
A very useful tool for this is the Data Integrity Maturity model from the ISPE GAMP Guide: Records & Data Integrity as shown below.

Level 1 is the point where no DI elements are in place, a situation rarely found. Level 2 is a common starting point where basic elements are in place but inconsistently performed. At Level 3, the policy and processes are well defined from the DI perspective. However, the infrastructure lacks follow-up. Level 4 shows that the defined processes are implemented according to the defined standard. Upon reaching Level 5, a proactive improvement program is in place allowing reflection on the current measures and implementation of necessary improvement actions.
GxP regulated industries depend on their data to ensure the quality of medicines and their related products. The quality of the data, through the full data lifecycle, is directly related to the quality of the decisions made to release products. Data integrity regulations assure that the quality of the data and the processes in which data is generated are regulated to guarantee the required quality.
Data integrity failures can lead to negative consequences, for example:
- Loss of GxP Authorization Certificate
- Product Quality Impact
- Patient Security Impact
- Recalls
- Financial Impact
Data integrity is all about the trustworthiness of the data and the quality of the final decisions taken on the data. Good Data Integrity practice means all controls are in place to prove the trust of data used for quality, efficacy, or safety decisions. Data integrity issues may break trust between regulators and industries as well as with the customers. According to the FDA, data integrity is now one of the most critical points of observation during audits.
DI Governance and Management Expertise
Data integrity supports business processes and the state of completeness, consistency, timeliness, accuracy, and validity which makes data appropriate. Legislation provides framework and guidelines help show the current situation and the way forward.
Advanced DI culture in a company ensures regulatory compliance, data security, and quality, a foundation that facilitates effective decision-making processes.
If you have questions about data integrity in your organization, contact us to discuss how we can help implement or maintain a compliant, efficient program to fit your unique needs.
TAGS: Life Science Consulting