In a Federal Register notice published today, the Food and Drug Administration (FDA) announced its intention to take enforcement action against misbranded or unapproved prescription products containing codeine, including codeine sulfate, codeine phosphate, and dihydrocodeine bitartrate. This notice comes four and half years after the approval of the new drug application (NDA) for codeine sulfate tablets, used to treat mild to moderately severe pain. In October 2009, the FDA issued warning letters to relevant companies manufacturing and/or marketing unapproved codeine sulfate tablets to inform them that they must stop producing and selling the drug. Though it is not clear if previous action was taken against manufacturers of drugs containing codeine phosphate or dihydrocodeine bitartrate, relevant products containing these active ingredients are still subject to the enforcement actions dictated by the notice. However, the FDA is allowing codeine-containing products that comply with the OTC Cold Cough monograph to remain on the market, which may cause confusion among manufacturers who may not know if their product complies with this monograph.
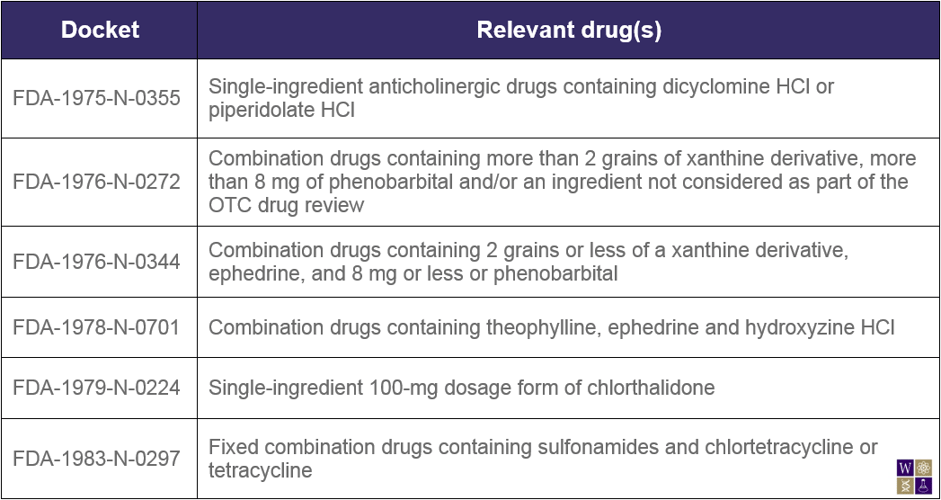
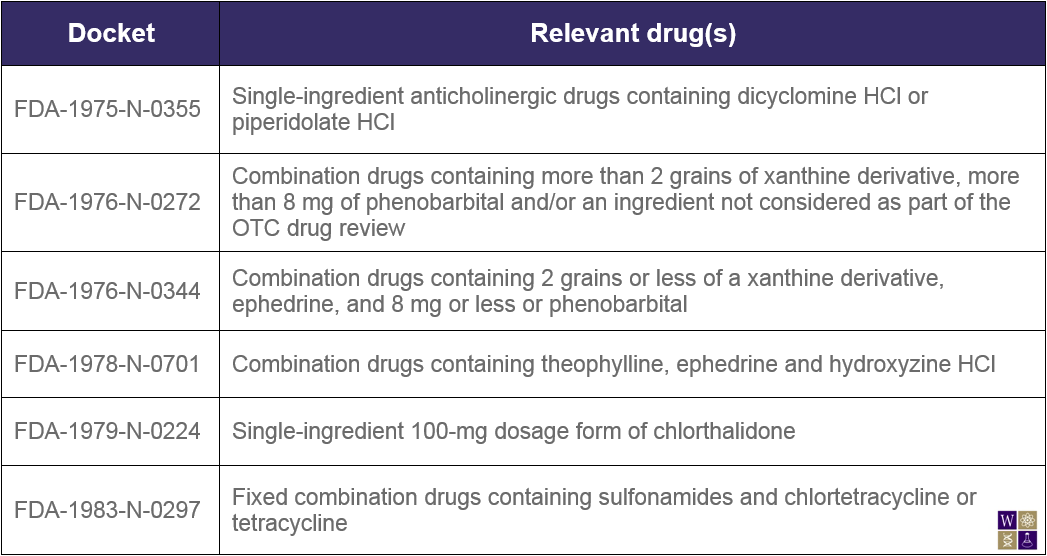
The Federal Register published another notice today with similar intentions, aimed at removing several DESI drugs from the market. Drugs marketed prior to 1962 did not have to be proven efficacious, only safe, to be approved. After the guidelines for drug approval were altered to contain efficacy requirements, the drug efficacy study implementation (DESI) program was designed to identify the effective drugs and remove the ineffective drugs from the market. A small number of drugs were deemed neither effective nor ineffective, and have therefore been allowed to remain on the market for many years, pending final FDA action. Specifically, the FDA refers to six unique dockets throughout this notice:
In July 2012, the FDA notified relevant companies that they had the opportunity to affirm or withdraw previously made hearing requests to discuss the regulatory status of these DESI drugs. The time period for responding to the July 2012 notice has elapsed, and therefore the FDA announces today that the hearing requests pertaining to the dockets in the notice are considered to be withdrawn. Additionally, the FDA states its intention to take enforcement action against those found shipping or manufacturing products covered in this notice as well as identical, related, or similar (IRS) products in these dockets.
The date of enforcement varies: for DESI drugs and some codeine drugs, the date of enforcement is today, January 10, 2014. Depending on the marketing start date in the United States and the current compliance with section 510 of the FD&C Act, which mandates companies to keep the Drug Registration and Listing System updated, enforcement may not start until up to 90 days from today for applicable codeine products.
Today’s notices affirm the FDA’s commitment to removing unapproved drugs from the market. While it is not advisable to continue with the production and sale of relevant codeine or DESI products, there are other options for companies to move forward in compliance with the FDA regulations rather than to abandon the drug products completely. Regulatory steps can be taken to apply for FDA approval of these products, therefore allowing their lawful sale within the United States. ProPharma Group is a regulatory consulting firm that specializes in the creation and implementation of high level regulatory strategy for drug companies and can assist both in interpreting the implications of this notice and developing a plan forward.