Maintaining compliance in the dynamic regulatory Chemistry, Manufacturing and Controls (CMC field can be quite a challenge.
A CMC regulatory dossier compliance assessment is a critical component and can minimize the risk of rejection and help avoid possible delays in the Health Authority’s review of your pharmaceutical product registration. Your compliance assessment can be performed prior to initial Marketing Authorization Application (MAA) or New Drug Application (NDA) submission and/or on a regular basis after approval. The frequency depends on the number of CMC changes to be introduced in the regulatory dossier and whether your product is intended to be rolled out globally.
In some cases, pharmaceutical companies may find that their procedures on the work floor are not compliant with the currently approved MAA/NDA or with the current guidelines. In these cases, effective remediation programs should be developed, implemented, and performed on a regular basis to assess the overall compliance status of the CMC information. By performing a regulatory dossier compliance assessment, also known as a gap analysis, deficiencies in the implementation of any site procedure or nonadherence to applicable guidelines can be identified, resolved, or rectified.
The outcome of this comparative review might require regulatory remediation of the MAA/NDA dossier for the identified gaps and discrepancies found in the registered information.
Understanding Compliance
Compliance means abiding by any and all applicable laws and regulations. Health Authorities (worldwide) are increasingly laying down rules that pharmaceutical products must comply with. Under Good Manufacturing Practices (GMPs), pharmaceutical products must be consistently produced and controlled to the quality standards appropriate to their intended use and as required by the MAA/NDA or product specification.
Compliance is a continuous process, not a snapshot. During a product’s lifecycle, companies are frequently being asked to demonstrate compliance with all applicable rules to avoid situations involving non-compliance, reputation damage, or supply issues.
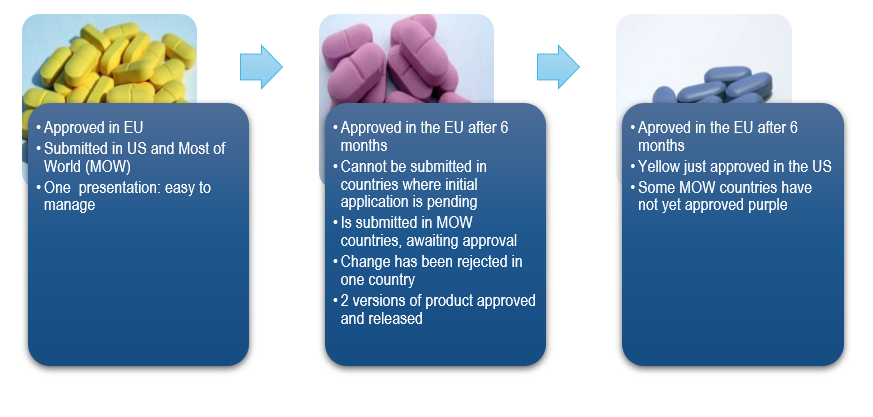
A Situation That Many Pharmaceutical Companies Will Recognize
During the lifetime of a pharmaceutical product, multiple changes occur which need to be reported and approved by all applicable, individual Health Authorities worldwide.
These changes include changes in:
- Chemistry – product characterization, release, and stability testing
- Manufacturing – facilities, equipment, utilities, raw materials, suppliers, and process
- Controls – analytical test methods (SOPs), batch records, training, auditing, and batch release
For each individual country, these changes may only be implemented after obtaining approval from the Health Authority. Depending on the country, the timelines for approval after submitting the variation dossier varies anywhere from one month to three years.

Challenges Facing Pharmaceutical Companies
Throughout the process, there are a number of challenges that many pharmaceutical companies encounter.
Some of these include:
- Keeping the regulatory dossier in compliance with the different requirements applied in each global market
- Providing regulatory impact assessment to identify which CMC changes must be submitted and/or approved first, or can be implemented immediately in global markets
- Keeping track of a product’s regulatory status, especially for pharmaceutical products registered in many countries
- Keeping all departments (Production, Quality Control, Quality Affairs) informed of updates related to the regulatory status of the pharmaceutical product
- Managing multiple CMC changes of many pharmaceutical products, including varying approval timelines in different countries requires having carefully planned strategies to ensure compliance
- Logistics related to multiple versions of one product, due to different regulatory status in countries
- Keeping track of and following-up on regulatory commitments
- Managing implementation of CMC changes after regulatory approval
Consequences of Non-Compliance
In some situations, a lack of compliance / non-compliance may lead to a number of undesirable situations, including:
- Issues with inspectorates
- Difficulty to regain compliance as submission of variations requiring present and proposed data can be a challenge
- Risk of out of stock as Qualified Persons should not release product which is not compliant with the MAA/NDA
- Where label information is involved, patient safety may be at stake
CMC Regulatory Dossier Compliance: What Sponsors Really Need
There is hardly any aspect of regulatory information management that is more complicated than CMC changes, since so many stakeholders and systems (quality management systems, change control systems, document management systems, labeling systems, regulatory tracking systems, and dossier publishing systems, etc.) are involved in the process. In addition, choices regarding the allocation of scarce resources need to be made which may result in the implementation of a prioritization strategy to be able to differentiate between the "nice-to-have" and "must-have" changes.
What are the "must-haves" to ensure your CMC regulatory dossier is compliant? Here is a checklist of items Sponsors really need to have in place:
- A change control system involving all stakeholders/supply chain as part of the quality system
- A change strategy to avoid multiple regulatory submissions
- Regulatory tracking as part of the quality system
- A communication process for regulatory status of submissions as part of the quality system
- Commitment tracking
- Trained personnel
How ProPharma Can Support You
When Sponsors have a hard time ensuring their MAA or NDA remains compliant, ProPharma can work with you quickly and efficiently to bring your product’s regulatory dossiers back into compliance by identifying where adjustments or improvements are necessary. Interested in learning more about our services and how we can help with all of your regulatory needs? Contact us today to learn how we can help you.
To learn more about our expert services, please read our blogs regarding this specific topic:
TAGS: Chemistry, Manufacturing, and Controls (CMC) Marketing Authorization Holder (MAH) New Drug Application Regulatory Sciences