October 20, 2022
October 20, 2022

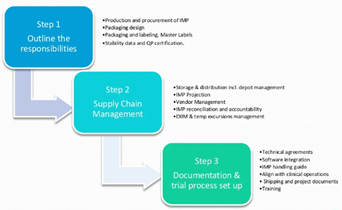
IMP Supply Management is a journey where GMP meets GCP and GDP. This journey includes finance, flow of products and documentation. How a company manages this flow can be referred to as IMP Supply (Chain) Management. The key objective is to Get The Right Drug To The Right Patient In The Right Time and Condition. IMP Supply Management is the management of operations that are involved in the procurement of raw materials, its processing of finished goods and distribution to the patient. See Figure 1 below.
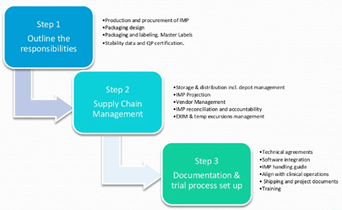
With the expansion of global services, ever-changing regulations, and patient focus at top of mind, it is paramount that identifying potential obstacles in the delivery of drug are recognized and managed as early and quickly as possible.
How hard can it be? Unfortunately, weakness in the drug supply chain can be anywhere and can be easily underestimated. The end goal is to deliver safe and effective drug to patients in a timely manner. As there are many posing threats to the Pharmaceutical Supply Chain, it has become more prevalent for companies to engage specialists and partners who can focus on developing an effective supply chain system. ProPharma Group is here to help!
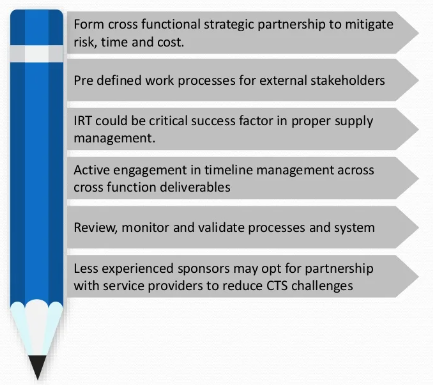
This Blog highlights key areas where ProPharma Group can support clients in risk mitigation, reducing long-term costs, reducing lead times, and minimizing errors by developing a robust Supply Chain Strategy.
Logistics is a broad area of expertise within Supply Management which encompasses many areas of knowledge and best practices. A logistical plan of where to develop, how to produce and deliver drug to patients should be done in the early stages of the supply chain strategy plan.
Most sites have limited storage capabilities – Planning of initial and restock supplies with time constraints in mind
Rules and Regulations of import/export of IMP and CTM vary from country to country. Supply Chain supports process-related issues to navigate challenging import and export requirements by collaborating with Regulatory and local vendors. Knowing how, when, and what documentation to prepare in case of Import/Export prior to the transfer of drug supply reduces frustration and can prevent fines and reduce lead times.
The most under-estimated area in supply planning is Lead Time. The amount of time it can take to source, manufacture, produce, and distribute a product to the end user can be several months. Timelines are crucial and top of mind when the goal is to help patients in need. Managing forecast versus demand, schedule changes, fluctuations in costs, and unexpected logistical issues can arise.
What to factor in:
| Forecasting Resupply | Lead Times |
| Expiry / Retest Dates | Time to receive API and all required excipients |
| Storage constraints at vendor | Time to receive components |
| Leadtime / Costs to manufacture batches | Time for Manufacturing (including getting in the manufacturing Queue) |
| Transportation / Customs costs for multiple shipments | Primary and Secondary packaging |
| Quantities to Order | Labelling |
| Distribution to Sites |
Packaging and labelling are two important parts of pharmaceutical products. Labelling is not only important for patient safety, but also correct labeling can help pharmacies avoid errors in relation to the storage of medicinal products. The obvious consequence of incorrect labelling is that a patient ends up with the wrong medicine or the wrong dose. In an extreme case, this could cause serious harm or even result in death of the patient. It is also important to avoid long lead-times and high costs. The design, ordering materials and having respect for lead-times while ensuring the label text is reviewed by minimum two people is important. Some factors to consider when deciding packaging & labelling design:
GMP Training
GDP Training
Challenges are unavoidable within Drug (IMP) Supply Chain, but clients can count on ProPharma Group to guide and support them through such challenges by implementing best practices and a supply chain strategy that can best handle their specific target and patient needs. Contact us today to connect with our team and tell us how we can help you achieve your goals.
TAGS: Life Science Consulting
October 10, 2012
One of the challenges to starting any User Requirement Specification (URS) is to envision a structure which can allow for traceability as the project continues. The attached sample URS is a starting...

August 30, 2022
Updates have been announced by FDA and for USP <1079>. In this blog we cover these changes. USP USP <1079> has a series of chapters on Good Storage and Distribution Practices. Chapter <1079> applies...