This article has been updated since its original publication date.
Navigating the complexities of Corrective Action / Preventive Action (CAPA) in the drug and medical device industries often poses a significant challenge, a sentiment echoed through real-life observation from ProPharma Quality and Compliance Consultants. Drawing from our wealth of experience in handling numerous CAPA-related projects, this article offers firsthand insights and effective strategies to successfully manage CAPA without being overwhelmed.
What is CAPA?
CAPA, an acronym for Corrective Action / Preventive Action, focuses on investigating, understanding, and rectifying discrepancies while preventing their recurrence, as outlined in the FDA's "Guidance for Industry: Quality System Approach to Pharmaceutical cGMP Regulations." CAPA is a crucial concept in the drug and medical device manufacturing industries. Here are the definitions:
Corrective Action (CA): Corrective Action is the immediate response and resolution taken to address and rectify a detected problem or nonconformity in a product, process, or system. It is implemented to eliminate the root cause of an issue and prevent its recurrence. Corrective Actions are typically reactive, responding to incidents that have already occurred.
In the context of drug and medical device manufacturing, Corrective Action could involve measures such as repairing equipment, amending documents, conducting process inspections, or rejecting products that do not meet quality standards. The goal is to address the immediate problem and prevent it from happening again.
Preventive Action (PA): Preventive Action involves proactively identifying and implementing measures to prevent the occurrence of potential problems, errors, or deviations before they happen. Unlike Corrective Action, which addresses existing issues, Preventive Action focuses on avoiding problems in the future. It aims to identify and eliminate the root causes of potential nonconformities.
In drug and medical device manufacturing, Preventive Action may include activities like training employees, modifying equipment, revising procedures, or making process improvements. The objective is to create a robust system that reduces the likelihood of errors or issues and enhances overall product quality.
CAPA Generation
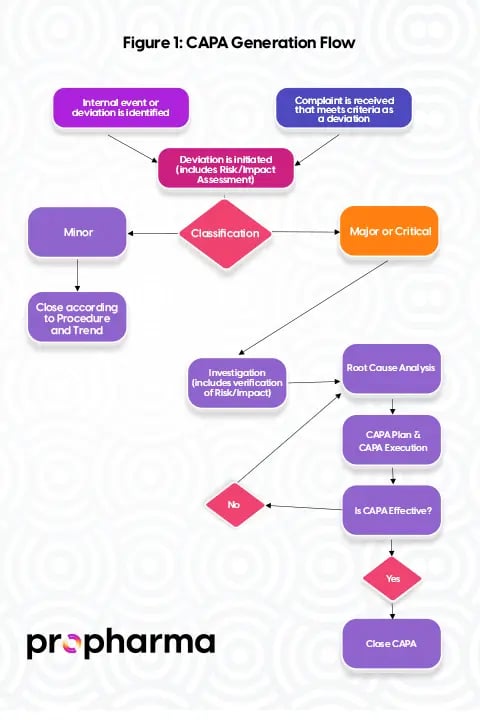
The generation of CAPA in drug and medical device manufacturing is a critical aspect of quality management. CAPA is typically initiated in response to various situations, and understanding how it is generated is essential for effectively managing and preventing issues. Figure 1 illustrates a simple process flow for how CAPA can be generated.
CAPA typically originates from a variety of situations, events, or circumstances within the drug and medical device manufacturing process. These situations trigger the need for a systematic approach to address and prevent issues. Common scenarios leading to CAPA generation include:
- Deviation Investigation: Deviations from established procedures or specifications are identified during manufacturing or testing processes.
- Out-of-Specification (OOS) Investigation: Test results fall outside the specified limits, indicating a potential quality or safety concern.
- Audit Observation: Internal or external audits identify noncompliance or areas for improvement in the manufacturing processes or quality management system.
- Process Improvement Need: Identification of opportunities for enhancing efficiency, reducing waste, or improving overall process performance.
- Customer Complaint Investigation: Reports of product defects, safety concerns, or dissatisfaction from customers.
- Adverse Event: Reports of unexpected, serious side effects or harm caused by the use of a drug or medical device.
- Ineffective CAPA Previously Assigned: Review indicates that a previously assigned CAPA did not effectively prevent the recurrence of the issue.
The Majority of CAPAs: Simple Errors and Documentation Issues:
A significant portion of CAPAs often arise from simple errors, underscoring the importance of clear communication and documentation in the manufacturing process. Common sources include:
- Documentation Errors: Ambiguities, inaccuracies, or omissions in written instructions, procedures, or specifications that lead to deviations.
- Personnel Errors: Mistakes made by individuals in following established procedures, resulting in nonconformities.
Addressing Errors
Errors often stem from unclear instructions or documentation. Ensuring clarity in quality documents can mitigate the risk of errors, emphasizing the need for complete and unambiguous communication. This simple optical illusion (Figure 2) provides a good visualization of how many errors occur. It emphasizes how there can be more than one way to perceive the same thing.
Drawing on insights gained from ProPharma consultants, it becomes evident that errors frequently result from unclear instructions or documentation. The imperative of ensuring clarity in quality documents emerges as a key strategy to mitigate the risk of errors. When creating your quality documents, instructions, directives, etc., ensure that they are clear and concise. For instance, use complete sentences that are non-ambiguous when writing a work instruction to avoid confusion or misinterpretation.
In addition to addressing errors reactively, implementing preventive measures is a proactive approach to error prevention. This involves:
- Root Cause Analysis: Performing thorough root cause analyses to identify the underlying factors contributing to errors and implementing measures to address them.
- Risk Assessment: Conducting regular risk assessments to identify potential sources of errors and their potential impact.
- Continuous Improvement: Embracing a culture of continuous improvement to evolve processes and systems in response to lessons learned from errors.
Importance of CAPA
Recognizing the significance of CAPA is crucial, driven by regulatory requirements, GMP compliance, system and process improvements, prevention of systemic problems, enhanced adherence to procedures, and cost reduction through error prevention.
By actively addressing errors and implementing preventive measures, organizations in the drug and medical device manufacturing industries can enhance the reliability of their processes, reduce the likelihood of deviations, and contribute to the overall quality and safety of their products. This comprehensive approach aligns with the principles of a robust CAPA system that seeks to correct issues and to prevent their recurrence.
How to Get Unburied: A Four-Part Approach to Effective CAPA Management
If you find your company submerged in a sea of overdue CAPAs, it's time to adopt a strategic approach to resurface and regain control. Our consultants use this four-part approach, which has proven effective in managing CAPA and reducing the burden of overdue actions.
- Awareness:
- Defined Procedures: Begin by verifying that your CAPA system procedures are well-defined and documented. Ensure that they address the requirements of quality system regulations.
- Metric Presentation: Present CAPA metrics at the outset of routine management or operational meetings. This not only emphasizes the importance of CAPA but also ensures that key stakeholders are informed.
- Management Support:
- Dissemination of Information: Determine if information regarding nonconforming products, quality problems, and corrective/preventive actions has been properly disseminated throughout the organization, including for management review.
- Top-Down Emphasis: Emphasize the importance of CAPA compliance from top management down. Lack of compliance is a notable source of FDA 483 observations, making it imperative for leadership to understand and support CAPA initiatives.
- Consolidation:
- Identification of Trends: Determine if sources of product and quality information highlighting unfavorable trends have been identified. Look for commonalities in multiple CAPAs that could be addressed with a shared solution.
- Common Fixes: Explore opportunities to consolidate multiple CAPAs with similar root causes into a single corrective or preventive action. This not only streamlines the process but also allows for a more comprehensive solution.
- Suspend/Inactivate:
- Document Review: Identify documents that can be inactivated to close out CAPAs. This may include obsolete or seldom-used documents.
- Immediate Reduction: Inactivating documents can yield immediate results by preventing the problem from recurring. It eliminates the need to revise documents that may never be utilized again, preventing wasted effort and ensuring correction before reuse.
By implementing these four steps, you can navigate the challenges of overdue CAPAs, identify patterns, and take strategic actions to streamline and optimize the CAPA management process. This approach reduces the burden of overdue CAPAs and contributes to a more efficient and proactive quality management system.
Sustaining CAPA Management:
Successfully reducing CAPA numbers is a commendable achievement, but the real triumph lies in maintaining the momentum gained and ensuring sustained compliance. Here are key strategies drawn from successful practices to help your organization foster a manageable and compliant level of CAPA over the long term:
- Incorporate CAPA into Employee Goals: Foster a sense of ownership and responsibility among employees by incorporating CAPA objectives into their individual performance goals.
- Designate a CAPA Coordinator: Assign a dedicated individual as a CAPA coordinator. This person serves as the central point of contact for CAPA-related activities, ensuring accountability and streamlined communication.
- Assign "Smart" CAPAs: Ensure that CAPAs are assigned strategically. A smart CAPA is a systematically planned and executed corrective and preventive action that is Specific, Measurable, Achievable, Relevant, and Time-Bound. This approach ensures that CAPAs are well-defined, realistic, meaningful, and implemented within specified timeframes, contributing to the overall effectiveness of a quality management system.
- Enhance Visibility to Department Supervisors: Be transparent with open CAPAs and make visible to supervisors. Department supervisors can play a crucial role in ensuring that CAPAs are effectively managed within their respective areas, driving a culture of compliance.
- Routine Reporting at Management Meetings: Keep up the routine reporting of open and past-due CAPAs at management meetings. Management awareness and engagement are vital for sustaining CAPA management efforts.
- Apply Real Fixes and Address Root Causes: When implementing corrective and preventive actions, focus on real fixes rather than temporary solutions. Continue to emphasize the importance of addressing root causes. This ensures that CAPAs are not merely reactive but contribute to a lasting improvement in processes.
- Evaluate Effectiveness and Success: Regularly evaluate the effectiveness of implemented CAPAs. Establish a feedback mechanism to capture lessons learned from CAPAs, enabling the organization to evolve its processes and systems over time.
CAPA as a Driving Force for Improvement
In conclusion, sustaining CAPA management involves embedding CAPA principles into the organizational culture and processes. By incorporating CAPA into employee goals, designating a coordinator, assigning "smart" CAPAs, enhancing visibility, maintaining routine reporting, applying real fixes, and evaluating effectiveness, your organization can foster a culture of continuous improvement and ensure that CAPA remains an integral and effective component of your quality management system.
Partner with CAPA Remediation Experts
When the complexities of CAPA management become too much to handle, it’s crucial to partner with seasoned professionals to swiftly regain control and establish sustainable processes. ProPharma’s team of experts have decades of experience spearheading successful CAPA-related projects. If your organization is grappling with CAPA challenges or falling behind, reach out to ProPharma. Contact us today and speak with an expert who can guide you toward CAPA excellence.
TAGS: Quality & Compliance Food & Drug Administration (FDA) Corrective and Preventive Actions (CAPA) Quality Assurance Life Science Consulting