Clinical Research Solutions
FDA Designates Empirically Based Bayesian Emax Models for Dose Finding as ‘Fit-For-Purpose’
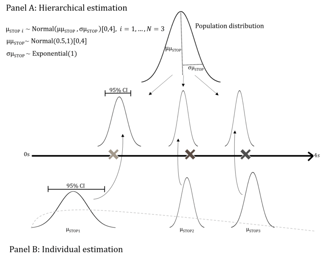
FDA Designates Empirically Based Bayesian Emax Models for Dose Finding as ‘Fit-For-Purpose’: On August 5, 2022, the U.S. Food and Drug Administration (FDA) designated ‘Empirically Based Bayesian Emax...

Clinical Research Solutions
FDA Issues FY2021 Report on the State of Pharmaceutical Quality
FDA Issues FY2021 Report on the State of Pharmaceutical Quality: The Office of Pharmaceutical Quality (OPQ) within FDA’s Center for Drug Evaluation and Research has published the fiscal year 2021...
Clinical Research Solutions
FDA Publishes Responses to Good Clinical Practice Inquiries
FDA Publishes Responses to Good Clinical Practice Inquiries: FDA oversees clinical trials to ensure they are designed, conducted, analyzed and reported according to federal law and FDA’s regulations....

Clinical Research Solutions
FDA publishes product-specific guidances to facilitate generic drug development
Today, FDA published a new batch of product-specific guidances (PSGs). PSGs provide recommendations for developing generic drugs and generating the evidence needed to support abbreviated new drug...

Clinical Research Solutions
Electronic Submission of Expedited Safety Reports From IND-Exempt BA/BE Studies Guidance for Industry
Guidance Document August 2022 This guidance provides instructions for the electronic submission of expedited individual case safety reports (ICSRs) from investigational new drug (IND)-exempt...

Clinical Research Solutions
EMA: Big data use for public health: publication of Big Data Steering Group workplan 2022-25
News July 28, 2022 The Big Data Steering Group set up by EMA and the Heads of Medicines Agencies (HMA) has published its third workplan that sets key actions to be delivered between 2022–25. The new...

Clinical Research Solutions
Clinical Pharmacology Considerations for Oligonucleotides
For the first time, the FDA has issued a draft guidance for industry on “Clinical Pharmacology Considerations for the Development of Oligonucleotide Therapeutics”. Oligonucleotides are short single...
Clinical Research Solutions
FDA Issues Final Guidance on Safety Considerations for Container Labels and Carton Labeling Design to Minimize Medication Errors
FDA has issued the final guidance titled “Safety Considerations for Container Labels and Carton Labeling Design to Minimize Medication Errors.” The recommendations for prescription drug and...

Clinical Research Solutions
Investigating Out-of-Specification (OOS) Test Results for Pharmaceutical Production - Level 2 revision
Guidance for Industry May 2022 Today, FDA is announcing revisions to the 2006 guidance “Investigating Out-of-Specification (OOS) Test Results for Pharmaceutical Production.” Specifically, this...
Clinical Research Solutions
2020 Trends in Biopharma
COVID-19 has been the ultimate Big Pharma catalyst, as well as the biggest thing to hit biopharmaceutical news pages in decades. On the industry side, however, investors and observers remain mindful...