Clinical Research Solutions
Understanding EMA and FDA Regulations on Nitrosamine Control
On September 26, 2019, the European Medicines Agency (EMA) released an advice to Marketing Authorization Holders (MAH) of human medicines to review their drug products on possible presence of...
Clinical Research Solutions
Are Your Compliance Obligations Being Properly Upheld? Avoid This Common Outsourcing Mistake!
Over the past several decades, the traditional approach to drug development and manufacturing has expanded to include the outsourcing of a range of functions from product development and testing, to...
Clinical Research Solutions
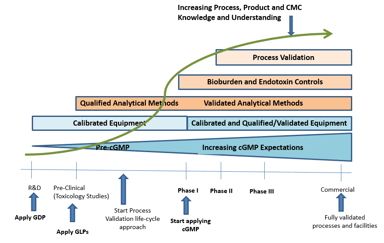
Pharmaceutical Tech Transfer Best Practices - A Quality Perspective
About 15 years ago, I was a project management director responsible for moving monoclonal antibodies (MABs) from Phase III clinical to commercial manufacturing. I had the distinct pleasure of working...
Clinical Research Solutions
A Roadmap to Authorization: Using Science to Prepare a MAA
As you move from Phase 3 clinical trials towards your Marketing Authorization Application (MAA), there are a number of critical steps that must be taken regarding how you interpret your clinical...
Clinical Research Solutions
Unlocking Success with Clinical Trial Safety Monitoring During a Pandemic
Earlier this year I wrote to you about US FDA March 2020 issuance of a new guidance for industry, Investigators, and Institutional Review Boards regarding the conduct of clinical trials during the...

Clinical Research Solutions
Meet the Expert: Matthew Weinberg
ProPharma Group has launched a “Meet the Expert” series introducing you to our experts from around the world. This series will help you get to know who we are, and how our colleagues work to improve...
Regulatory Sciences
10 Things You Need to Know about FDA’s Final Rule on Importing Prescription Drugs from Canada
Last week, FDA issued a final rule regarding the importation of certain prescription drugs from Canada. This action was taken by the agency as part of the Safe Important Action Plan, and was done in...

Clinical Research Solutions
How to Prepare for Laboratory Partner Selection during CBD Product Development
The interest in developing consumer products or therapies derived from Cannabis or CBD is continuously growing. As these new products come to market, there is increasing need to comply with...

