
Clinical Research Solutions
Meet the Expert: Marla Scarola
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...
Clinical Research Solutions
Understanding The Medical Device Single Audit Program
The Medical Device Single Audit Program (MDSAP), initiated by the International Medical Device Regulators Forum (IMDRF), created a global approach to auditing and monitoring the manufacturing of...
Clinical Research Solutions
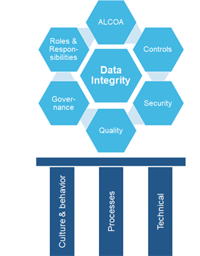
Creating Efficiencies with Data Integrity
Over recent years relations with data and its weight have changed significantly; consequently, an assessment of Company Data Integrity is becoming one of the key metrics of corporate functionality...
Clinical Research Solutions
Innovation and Rapid Growth: A Double-Edged Sword
Every industry has a distinct set of obstacles to overcome, but it’s no secret that the life sciences industry encounters more speedbumps than most. Market cycles and product failures are expected,...
Clinical Research Solutions
Fundamentals of Data Integrity for ATMP Development
Advanced Therapy Medicinal Products (ATMPs), or Cell and Gene Therapies (CGT), have the incredible potential to cure devastating illnesses, such as cancer, on a more personalized level. But, due to...
Clinical Research Solutions
FDA Guidance on Gene Therapy Products: RCR Testing and Monitoring – Focus on Patient Monitoring
In July 2018, FDA issued a draft guidance document entitled "Testing of Retroviral Vector-Based Human Gene Therapy Products for Replication Competent Retrovirus During Product Manufacture and Patient...
Clinical Research Solutions
What to Expect From an FDA Inspection, Part 2
In this continuation from “Preparing for an FDA Inspection” I will discuss what I have witnessed as a typical outline of events for FDA inspections. Prior to setting foot at the facility, the FDA...

Clinical Research Solutions
Medical Monitoring: Learning from the Past & Looking Ahead
Reflecting on the 1937 Elixir of Sulfanilamide incident we can clearly appreciate the importance of clinical trials and monitoring patient safety today. For those that may not know, Sulfanilamide was...

Clinical Research Solutions
Meet the Expert: Anchal Choudhuri
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...
Clinical Research Solutions
Preparing for an FDA Inspection, Part 1
The ultimate goal of the U.S. Food and Drug Administration is to protect the public welfare and protect consumers from unsafe products. Regulatory investigators are responsible for enforcing...