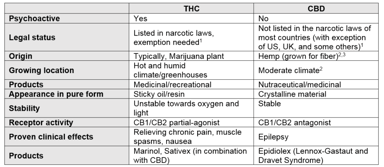
7 Tips to Help You Prepare for Compliance in a Post COVID-19 World
Yes, there is a light at the end of the long, dark COVID-19 tunnel, and people’s lives will return to a state of normalcy. However, what will the new state of normalcy look like in a post COVID-19...
Meet the Expert: Robert Beall, PMP
ProPharma Group has launched a “Meet the Expert” series introducing you to our experts from around the world. This series will help you get to know who we are, and how our colleagues work to improve...

Meet the Expert: Daniel Wong
ProPharma Group has launched a “Meet the Expert” series introducing you to our experts from around the world. This series will help you get to know who we are, and how our colleagues work to improve...
Clinical Research Solutions
4 Common Bottlenecks to Avoid in the Development of Biopharmaceuticals
If you are involved in early development of biopharmaceuticals, have you ever experienced serious delays because of problems arising from tech transfer, from the first pilot scale batches not meeting...
A Roadmap to Authorization: Using Science to Prepare a MAA
As you move from Phase 3 clinical trials towards your Marketing Authorization Application (MAA), there are a number of critical steps that must be taken regarding how you interpret your clinical...

Meet the Expert: Matthew Weinberg
ProPharma Group has launched a “Meet the Expert” series introducing you to our experts from around the world. This series will help you get to know who we are, and how our colleagues work to improve...
Regulatory Sciences
10 Things You Need to Know about FDA’s Final Rule on Importing Prescription Drugs from Canada
Last week, FDA issued a final rule regarding the importation of certain prescription drugs from Canada. This action was taken by the agency as part of the Safe Important Action Plan, and was done in...

Clinical Research Solutions
Biosimilars: How the Approval Process Differs from a Standard ANDA
Biosimilars, by their very nature, are different from generic drugs. The core difference is that generic drugs are chemically synthesized, while biosimilars are grown in complex living systems....
505(b)(1) vs. 505(b)(2): Choosing the Appropriate Regulatory Pathway
Because the FDA is an agency established by a federal law, there are clearly defined pathways along which a drug can be approved. Deciding on the appropriate regulatory pathway for your drug...
6 Quick Tips for Excel Spreadsheet Validation
Are you using Microsoft Excel spreadsheets for GxP activities? Are these Excel sheets used again and again, as a template? Ever thought about validating your spreadsheet templates? Is the person who...

Meet the Expert: Hannah Hunter
ProPharma Group has launched a “Meet the Expert” series introducing you to our experts from around the world. This series will help you get to know who we are, and how our colleagues work to support...
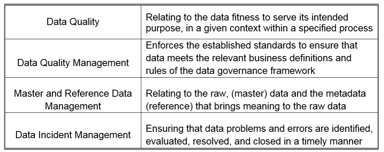
Just How Mature Is Your Data Lifecycle / Data Management Function?
The attention of regulatory agencies continues to focus on data integrity, as observed by the increase of FDA observations over the course of the last few years. Having a proper data lifecycle / data...
Clinical Research Solutions
Get the Most Out of Your GMP Effectiveness Checks
We work in a highly regulated industry. Whether you are associated with the manufacturing of a drug, a biologic, or a device, you understand the importance of those regulations on the safety and...
Utilizing an Agile Framework When Implementing ATMPs
Who says you can’t teach an old dog a new trick? Having spent the last 25+ years in small molecule, large molecule, medical devices, I have spent a lot of time planning and executing everything from...