eQMS: Your Questions, Answered
If you work in a regulated industry, you’ve most likely heard the term eQMS or enterprise quality management system. But you may be wondering what is it? Why do I need it? And how do I choose the...
How to Adopt an eQMS in 3 Simple Steps
A recent survey showed that 33% of the organizations surveyed use paper quality management systems; 60% use some paper and some digital; and 7% use no QMS yet. (source: Gartner peer insights) Quality...

Meet the Expert: Daniel Solorio
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...

Meet the Expert: Hanna Edling
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...
Have an FDA Submission on Your 2021 To-Do List?
At the start of every year, we all have these grand plans of everything we plan to accomplish. It is a fresh start to really get stuff done and we have a full 12 months to do it all. However,...

Meet the Expert: Bram Lardée
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...
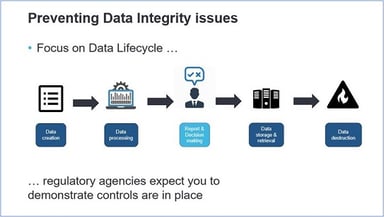
Measuring the Maturity of Data Integrity
We live in a world of data: there’s more of it than ever before, in a ceaselessly expanding array of forms and locations. Besides this, most people in their organizations are not always aware of data...

Meet the Expert: Marla Scarola
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...
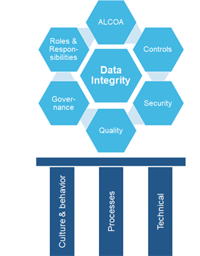
Creating Efficiencies with Data Integrity
Over recent years relations with data and its weight have changed significantly; consequently, an assessment of Company Data Integrity is becoming one of the key metrics of corporate functionality...
Clinical Research Solutions
Innovation and Rapid Growth: A Double-Edged Sword
Every industry has a distinct set of obstacles to overcome, but it’s no secret that the life sciences industry encounters more speedbumps than most. Market cycles and product failures are expected,...
FDA Guidance on Gene Therapy Products: RCR Testing and Monitoring – Focus on Patient Monitoring
In July 2018, FDA issued a draft guidance document entitled "Testing of Retroviral Vector-Based Human Gene Therapy Products for Replication Competent Retrovirus During Product Manufacture and Patient...

Meet the Expert: Anchal Choudhuri
Our “Meet the Expert” series introduces you to our team of experts around the world. This “behind the curtain” view will help you get to know who we are on a professional and personal level, and...
Clinical Research Solutions
Tips for Effective Commercialization in Europe From A Quality Perspective
The implementation of a robust Quality Management System (QMS) is a key success factor for clinical development with the goal of reaching marketing authorization. As important as your QMS is during...
Clinical Research Solutions
Post-COVID Challenges with Investigation Backlog: Are You Ready for the Inevitable?
In early 2020, while COVID-19 was wreaking havoc on public health and safety, the FDA took the unprecedented step of postponing domestic and foreign inspections. The FDA’s risk-based inspection...
Clinical Research Solutions
The Anniversary We Didn’t Want: One Year of COVID-19 Milestones
On March 11, 2020, after months of researching, strategizing, and meeting with various leaders and medical experts globally, the World Health Organization (WHO) declared COVID-19 to be a global...
What It Takes to Be Successful with GDUFA in 2021
GDUFA II was signed into law in 2017 effectively reauthorizing GDUFA I for fiscal years 2018 through 2022. The continuing goal of the amendment is to facilitate timely access to high-quality,...