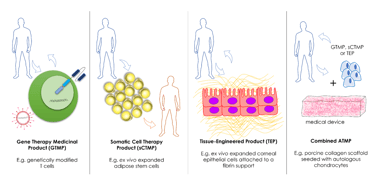
Clinical Research Solutions
Clinical Trial Good Clinical Practice (GCP) Audits – Are you ready?
Good clinical practice (GCP) is an international ethical analysis and scientific quality standard for designing, conducting, and auditing clinical trials that involve the participation of human...
Clinical Research Solutions
Ban on Titanium Dioxide (E171) on the EU Food Market: What Are the Consequences for Medicines?
Use of Titanium Dioxide in the EU Food Market In 2021, the European Food Safety Authority (EFSA) investigated the safety of the white coloring agent titanium dioxide (TiO2) and concluded that the...

Clinical Research Solutions
Be Careful What You Ask For (Prior to Consent)
According to FDA’s clinical trial regulations (21 CFR 50.20, 312.60 and 812.100), clinical investigators are responsible for protecting the rights, safety, and welfare of subjects during a clinical...

Clinical Research Solutions
Your Vendor Audit Program: On-site or Remote / Virtual?
It is quite common that a sponsor company will outsource services to external vendors, whether for additional expertise, remote locations, or simply due to lack of availability of resources within...
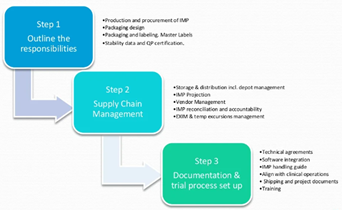
Clinical Research Solutions
Clinical (IMP) Drug Supply…It’s Complicated
Things to consider and how to ease the process IMP Supply Management is a journey where GMP meets GCP and GDP. This journey includes finance, flow of products and documentation. How a company manages...
Clinical Research Solutions
What You Need to Know About Developing Vaccines
An unlikely beacon of hope from the otherwise disastrous Covid pandemic, may come in the form of renewed attention towards approaches to vaccine development. The Importance of Vaccines The...

Clinical Research Solutions
Meet the Expert: Sharon Charles
Our "Meet the Expert" series introduces you to our team of experts around the world. This "behind the curtain" view will help you get to know who we are on a professional and personal level, and...

Clinical Research Solutions
How to Comply with the Nitrosamine Regulations for Your New Drug Product Marketing Applications
Introduction: Are you in the development phase for your medicinal product? Have you assessed your manufacturing processes with respect to the requirements for investigating the potential presence of...

Clinical Research Solutions
USP and FDA Propose Updates to Good Storage and Distribution Practices
Updates have been announced by FDA and for USP <1079>. In this blog we cover these changes. USP USP <1079> has a series of chapters on Good Storage and Distribution Practices. Chapter <1079> applies...