August 12, 2015

August 12, 2015

Do you find yourself in one of the following situations?
+ Your equipment has broken down due to frequent usage or wear and thus requires repair. You realize that once repaired, any equipment will need to undergo requalification in order to make sure it continues to work accurately.
+ Your new equipment has been purchased and as much as you would like to just plug it in and start using it, you know it first needs to be properly qualified for its intended use.
+ You want to make room in your lab and decide that you are going to move that HPLC to the next bench… if only it were that easy.
If so, your scenario requires that such equipment be qualified in a compliant manner.
Often times, when a company has a major lab equipment qualification also known as Analytical Instrumentation Qualification (AIQ) project to complete, they simply proceed with the intention of qualifying the instrumentation, through in-house or hired validation consultantscontractors. The result is usually a reactionary approach to problems as they arise, whereas instead laboratory management should assess their systems and devise a methodical approach to ensure they are ready for an AIQ project.
What is the purpose of Analytical Instrumentation Qualification?
Analytical Instrument Qualification is necessary because it assures instruments provide data that is valid, reliable and reproducible. All instruments used in the pharmaceutical industry are required to always provide consistent and accurate data. Accuracy, precision and integrity of the instrument are established through qualification and validation. This is very important for all lab equipment, and for everyone in this industry who relies on the data produced by the instruments.
An Approach:
Qualification should provide documented verification that the parameters defined as critical for operation and maintenance are adequately controlled. It is essential that the qualification is practical and achievable, that it adds value to the project and is concentrated on the critical elements of the system under qualification.
+Plan
Prepare a Project Validation Master Plan (VMP) and/or Equipment Qualification Plan (QP). Depending on the level of specificity, a section of the Validation Master Plan should cover the qualification of facilities, utilities, equipment and of course, analytical instrumentation.
The objective of this validation plan is to outline the requirements that will demonstrate and document that all components, control system(s) and functionality associated with each instrument to be qualified are appropriate for cGMP-regulated processes. The qualifications outlined are to be based on in-house policies and procedures and applicable regulations, guidelines, and accepted industry practices for validation.
+Assess
The first requirement is to assess the complexity of the equipment to ascertain that the scope of the proposed validation is adequate to verify that all aspects of the of the user requirements specification are verified as being satisfied. It is recommended that a risk assessment approach is used to define the depth of qualification. There should always be a reasonable proportionality between risk for the product quality, the amount of measures to be taken and documentation to be prepared (e.g. stage of manufacture, regulatory status and intended use).
Execute the Risk Assessment Process to identify which equipment items require qualification and the level of the same. The risk assessment process should be carried out systematically on every instrument. Refer to the flow diagram in figure 1 below; if any answer to the six (6) questions is a ‘yes’ then the equipment is within the scope of your Equipment Qualification Plan and must be validated.
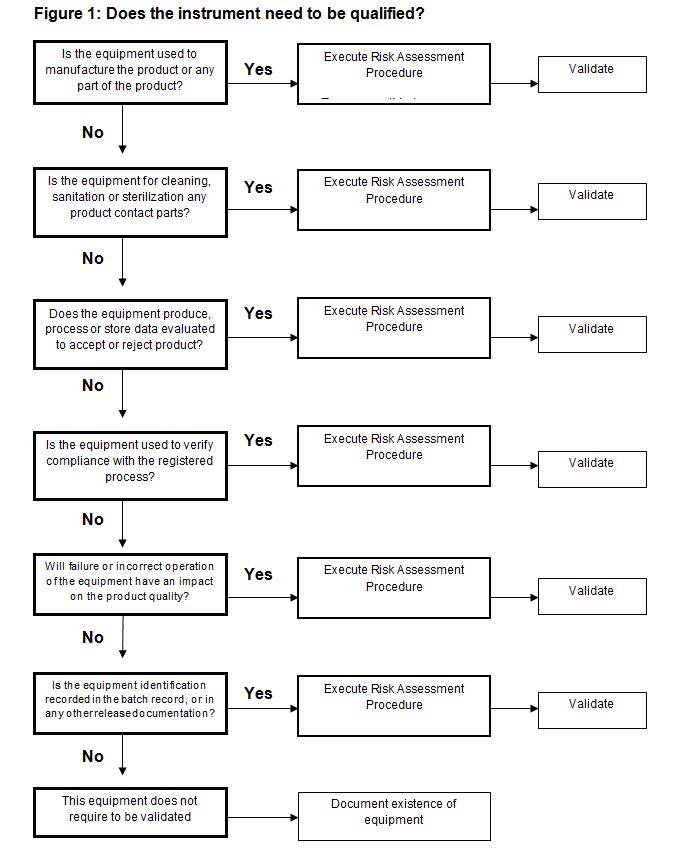
The next steps include calculating the degree of risk for each instrument, assessing documentation, selecting team members, and developing a schedule of deliverables. Each of these topics will be covered in the final segment of this blog later this month.
TAGS: Analytical Instrument Qualification Life Science Consulting
March 12, 2015
Moving an operational Quality Control Laboratory is all about maintaining the support of production testing, ensuring physical integrity of the instrumentation being moved, and qualification of the...

June 20, 2024
What is a LIMS? At first, a Laboratory Information Management System (LIMS) sounds like something that would be useful in any lab. Isn't the purpose of a lab to produce information, and shouldn’t...