Inspection Readiness
Our team of experts are equipped to guide you through every step of any Inspection your firm is facing, with experience and expertise.
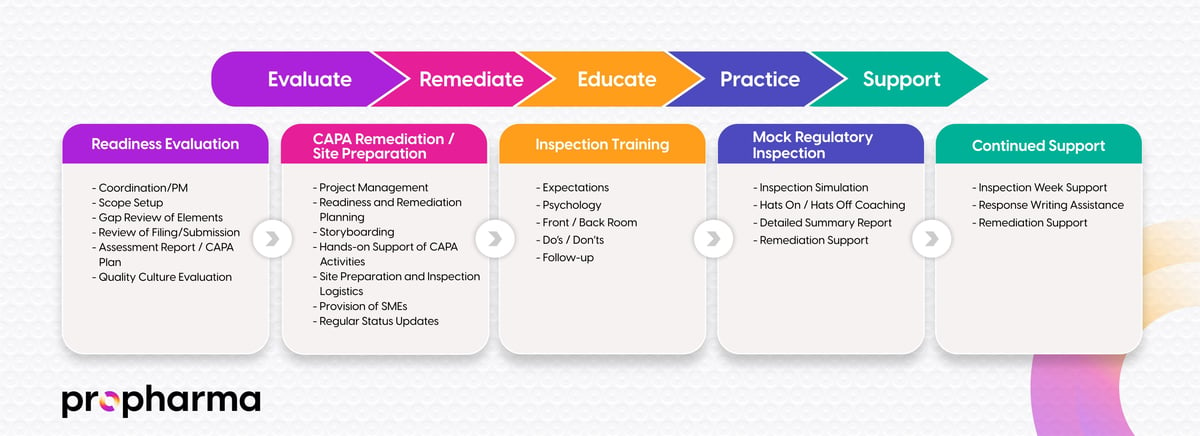
We ensure that every step towards Inspection Readiness is carefully calibrated to your needs. Our method is backed by tangible tools and training programs developed from the ground up, tailored not just to your product, but to your organization's unique scale and scope. This approach allows you to maintain focus on running your business, while we dedicate ourselves fully to your Inspection Readiness, making it our singular priority.
Secure Your Regulatory Inspection Success
On average a new product takes approximately 10-15 years to develop, whether it is a biologic, a pharmaceutical product, a medical device, or a combination thereof.
- Can you risk the time and resources you invested in development by ignoring the importance of Inspection Readiness for a new product?
- Can you risk having an existing product off the market for remediation activities following a For Cause Inspection?
- Can your patients?
ProPharma's expertise covers all aspects of Inspections. Inspection Readiness Evaluation, Project Management, your Inspection Program/Process, Logistics, and Training – are just some of the most important areas of Inspection Readiness. Our expertise can become your greatest asset. We are eager to become your partner in navigating this critical process.


PAI Readiness
Pre-approval Inspections are one of the most critical inspections any Biologic, Pharmaceutical or Medical Device firm will undertake. Failure to meet FDA expectations can have a huge impact on organizations, including:
- Delays to product launch.
- Thousands, if not millions of dollars, in remediation costs.
- Lost market share.
- Loss of a firm's credibility.
PAI readiness is critical, and ProPharma is an industry leader in providing PAI Readiness to our clients.
BIMO Readiness
BIMO inspection readiness is essential for sponsors to ensure compliance with FDA regulations, protect data integrity, and uphold patient safety standards. By preparing thoroughly, sponsors:
- Ensure compliance with FDA regulations.
- Protect data integrity.
- Uphold patient safety standards.
- Help avoid costly delays.
- Prevent potential compliance issues.
- Facilitate smoother FDA inspections.

To date, we have had a 100% success rate on PAI Readiness projects for our clients
News & Insights

April 28, 2025
Advances in AI for Digital Transformation: Insights from BioIT World 2025
Discover how advances in AI are transforming biopharma, from predictive models to data integration, and explore the benefits and challenges of AI in the industry at BioIT World 2025&d

April 22, 2025
The Key to Understanding Pricing and Reimbursement in the Nordics
Discover the essential steps to navigate the complex Pricing and Reimbursement process for pharmaceuticals in the Nordic countries, ensuring successful market access for your drug.

January 31, 2025
ProPharma Recognized for AI Excellence at ECCCSA
ProPharma wins Silver at ECCCSA for AI innovation in Medical Information, enhancing efficiency and quality in delivering accurate medical information.

October 21, 2024
ProPharma Appoints Brian Tuttle as Chief Financial Officer
ProPharma appoints Brian Tuttle as CFO to drive financial strategy and accelerate global growth, leveraging his 20 years of life sciences industry experience.

January 31, 2025
ProPharma Recognized for AI Excellence at ECCCSA
ProPharma wins Silver at ECCCSA for AI innovation in Medical Information, enhancing efficiency and quality in delivering accurate medical information.

October 9, 2024
ProPharma Receives 2024 CPHI Regulatory and Compliance Award
ProPharma wins CPHI Pharma Award for excellence in regulatory and compliance innovation, enhancing efficiency and accelerating market access for life-saving therapies.

June 10, 2022
The Cost of Poor Project Management
Project Management isn’t for the faint of heart. There is a shockingly high rate of project failure… but on the other hand, great project management can be a key differentiator that leads a company...

August 31, 2022
Successfully Passing MHRA Inspections for Overseas Manufacturing Sites
ProPharma offers GMP and GDP compliance services from clinical development to commercial distribution of the products' lifecycle. ProPharma’s Compliance and Quality team completed the first on-site...
News & Insights


